Gram stain is the most widely used standard procedure in microbiology that is used to classify bacteria according to their cell wall composition.
It distinguishes the bacteria into two major groups; Gram-positive and Gram-negative bacteria. see : Difference between gram-positive and gram-negative bacteria.
It is a valuable diagnostic tool in both research and clinical settings. It helps in the characterization and identification of bacteria.
Historical background
The gram staining procedure was first developed by a Danish Bacteriologist and Physician, Hans Christian Gram in 1884 while he was working in Berlin when he used the Gram staining technique to visualize Klebsiella pneumoniae in the lung tissue section of people who died because of pneumonia infection. Before this, the available staining method was designed by Robert Koch for visualizing tubercle bacilli.
Gram didn’t use safranin as a counterstain in the original procedure. The nuclei of eukaryotic cells in tissue samples were left unstained, while the cocci were stained blue/violet in the lungs of those who died of pneumonia. Later, Carl Weigert, a German Pathologist, used safranin as a counterstain.
Principle of Gram staining technique
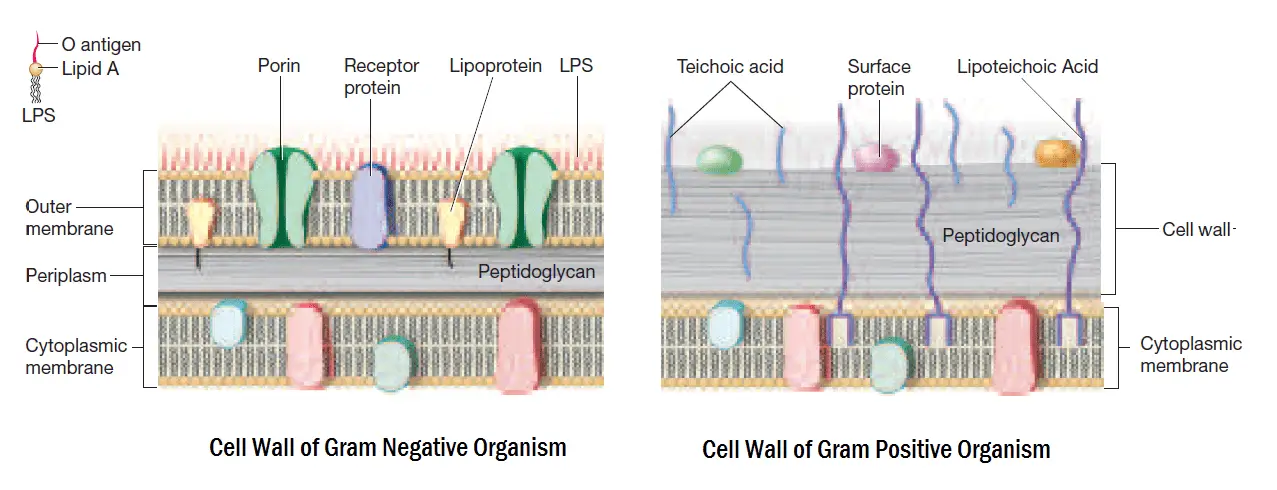
The basic principle of the gram staining technique involves the ability of the cell wall to retain the primary stain. The cell wall structure will determine whether the organism is gram-positive or gram-negative. Gram-positive bacteria have higher peptidoglycan content, whereas gram-negative bacteria have more lipid content in the cell wall.
When stained with a primary dye and fixed by a mordant, some bacteria can retain the primary stain by resisting decolorization, while others get decolorized by a decolorizer. Those bacteria which retain the primary color are called Gram-positive bacteria, while those bacteria which get decolorized and then get counterstained are called Gram-negative bacteria.
- Crystal violet (CV) molecule splits into CV+ and Cl– ions in aqueous solutions. These ions will penetrate the cell wall and cell membrane of both cells (gram-positive and gram-negative).
- The CV+ ions stain the cells purple color because of their interaction with the components of bacterial cells that are negatively charged.
- Iodine (I), used as mordant, interacts with CV+ and forms large complexes of crystal violet and iodine (CV–I). The process will occur in the outer layers and within the cytoplasm.
- When a decolorizer such as alcohol or acetone is added, it will further interact with the lipid content of the cell membrane of both gram-negative and gram-positive bacteria.
- Gram-negative bacteria will lose their outer membrane leaving the peptidoglycan layer exposed.
- A thin layer of Gram-negative bacteria will leak the large crystal violet and iodine (CV–I) complexes from the cell.
- On the other hand, a Gram-positive organism will be dehydrated from alcohol treatment. This will close the pores inside the cell wall and prevent the dye from exiting the cell.
- The large complexes of CV–I will be trapped within the Gram-positive cells. It is due to the presence of the thick and multilayered peptidoglycan.
- After decolorization, the Gram-negative bacteria will lose the purple dye and gram-positive bacteria will retain it.
- Counterstain, usually safranin or fuchsin, will give these Gram-negative cells a pink or red color.
Difference between Gram-staining and acid-fast techniques
Following are the differences between the gram-staining and acid-fast techniques.
| Gram-staining technique | Acid-fast technique |
| It is a staining technique for identifying bacteria. Violet dye, decolorizing agent, and secondary dye are used. | It is a technique in which we use differential stains to identify acid-fast organisms like Mycobacterium. |
| It characterizes bacteria into two large groups. | It is used to differentiate Gram-positive bacteria. |
| Identifies in the basis of different types of the cell wall. | Identifies based on mycolic acid in the cell wall. |
| Differentiates into Gram-positive and Gram-negative. | Differentiates into acid-fast and non-acid-fast bacteria. |
| The primary stain is crystal violet. | The primary stain is carbofuchsin. |
| The counterstain is safranin. | The counterstain is methylene blue. |
| Gram-positive appear in blue and Gram-negative in red color. | Acid-fast bacteria in red color and non-acid fast in blue color. |

Specimen
Various specimens can be used in this technique. Some of the commonly used specimens are following
- Urine
- Ascitic fluid
- Sputum
- Cerebrospinal fluid (CSF)
- Blood
- Swabs from throat, rectum, wound, nostrils, cervix, etc
- Synovial fluid
- Pleural fluid
Reagents
Reagents used in this technique are following
- Crystal-violet stain
- Gram’s Iodine
- Decolorizer (ethanol)
- Safranin
- Water
Clinical uses of Gram-staining
- Provides preliminary clue in anaerobic culture.
- Identifies Candida and Cryptococcus Spp.
- Classifies the bacteria into two groups, gram-positive and gram-negative bacteria.
- Determines the cell morphology, size, and arrangement.
- Identifies fastidious organisms like Haemophilus.
- Helps in initiating empirical treatment with broad-spectrum antibiotics. It can be initiated before recieving the culture report.
Specimens used for Gram-staining technique
The specimens used for this technique are
- Stool
- Urine
- Blood
- Tissue
- Sputum
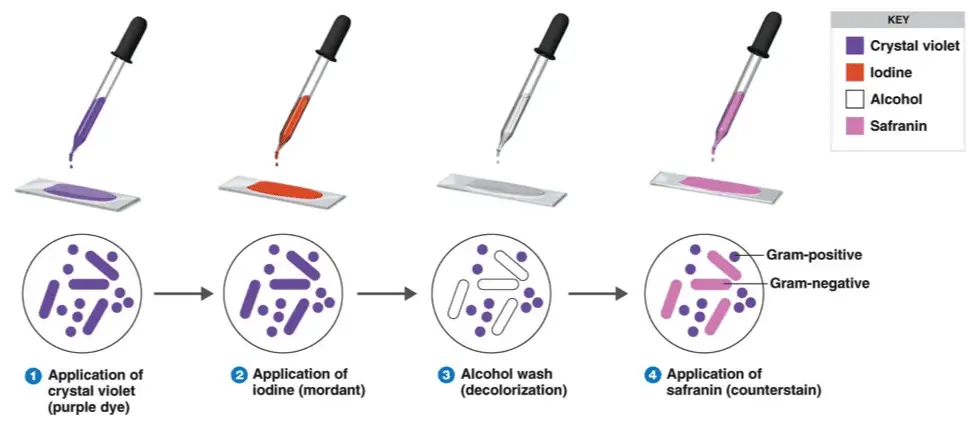
Four basic steps
- Staining a heat-fixed smear with primary crystal violet stain
- Addition of a mordant (Gram’s iodine)
- Rapid decolorization with alcohol or acetone
- Counterstaining with dye, usually safranin
Steps in Gram-staining technique
- Take a grease-free dry slide. And wipe the bottom of the biofilm slide clean.

- Clean top edges of the slide about 2mm

- Build up a ridge of petroleum jelly on the top and bottom of a cover slide
- Cover slip with petroleum jelly

- Put the biofilm on slide with cover slip

- Stain with primary crystal violet stain and incubate for almost 1 minute.

- Rinse the slide with a stream of water for almost 5 seconds to remove unbound crystal violet.

- Add mordant (Gram’s iodine) for 1 minute. Rinse the slide with distilled water
- It forms a complex between crystal violet and iodine.

- Wash with 95% decolorizer (acetone or ethyl alcohol) for almost 3 seconds

- Rinse with a gentle stream of water.
- It will dehydrate the peptidoglycan layer, shrinking and tightening it.
- The alcohol will decolorize the sample if it is Gram-negative, removing the crystal violet.
- However, if the alcohol remains on the sample for too long, it may also decolorize Gram-positive cells.

- Add the secondary stain to the slide and incubate it for 1 minute.

- Wash with a stream of water for 5 seconds.

- Observe under a microscope.
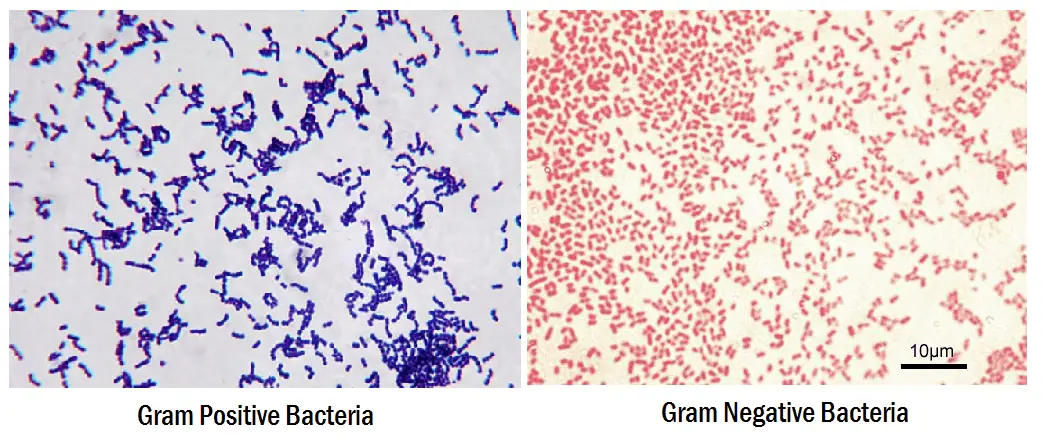
- Gram-positive bacteria will retain the primary stain and will not take the secondary stain, and looks violet/purple color under a microscope.
- If it is Gram-negative bacteria, it will not retain the primary stain. Still, it will take the secondary stain, and appears red color when viewed under a microscope

Errors during the gram-staining procedure
- Prolonged decolourisation
- Excessive counterstaining
- Excessive heat while fixation
- Excessive washing between steps
- Insufficient iodine exposure
- Low concentration of crystal violet
Artifacts in gram-staining
- Dirty glass slides
- Gram-staining reagents contamination
- Contaminated water used to rinse slides
Requirements and preparation of reagents
Primary Stain : Crystal violet
Solution A :
- Crystal violet = 2 gm
- Ethyl alcohol= 20 ml
Solution B :
- Ammonium oxalate = 0.8 gm
- Distilled water = 80 ml
Mix solution A and B. Keep for 24 hours and filter. Store in an amber-colored bottle.
Mordant : Gram’s Iodine
- Iodine = 1 gm
- Potassium iodide = 2 gm
- Distilled water = to 100 ml
Mix and Store in an amber-colored bottle.
Decolorizer : 95% Ethanol or 1:1 acetone with ethanol
- Acetone = 50 ml
- Ethanol (95%) = 50ml
Counterstain: safranin
- Safranin O = 0.34 gm
- Absolute alcohol = 10ml
- Distilled water = 90ml
Mix, filter, and store in an ambered colored bottle.
Interpretation of Gram staining
The staining results of gram stain are as follows:
- Gram-positive will be dark-purple color
- Gram-negative will be pale to dark red color
- Yeasts will be dark purple color
- Epithelial cells will be pale red color
Examples of Gram-positive Organisms
Bacillus, Nocardia, Clostridium, Propionibacterium, Actinomyces, Enterococcus, Corynebacterium, Listeria, Lactobacillus, Gardnerella, Mycoplasma, Staphylococcus, Streptomyces, Streptococcus, etc.
Examples of Gram-negative Organisms
Escherichia, Helicobacter, Hemophilus, Neisseria, Klebsiella, Enterobacter, Chlamydia, Vibrio, Pseudomonas, Salmonella, Shigella, etc.
Animation of gram staining
Frequently Asked Questions
Q1. What are the primary colors used for the Gram-staining technique?
Q2. Which counterstain is used in the Gram-staining technique?
Q3. Is there any risk to doing a gram-staining test?
Q4. How is Gram-staining performed?
We have to introduce a dye to bacteria. If bacteria have a thick peptidoglycan cell wall, it absorbs the stain and turns purple-color. And we can say that it’s positive for peptidoglycan. If it’s not turned into purple color, the test is negative for peptidoglycan.
Q5. Can we use the gram-staining technique for Eukaryotes?
Q6. Can we gram-stain a virus?
Q7. Why can’t a mycobacterium be stained with Gram-staining?
Q8. Which bacteria can’t be stained with Gram-staining?
References
- Coico, Richard. “Gram staining.” Current protocols in microbiology 1 (2006): A-3C.
- Hucker, George J., and Harold Joel Conn. “Methods of Gram staining.” (1923).
- Tripathi, Nishant, and Amit Sapra. “Gram Staining.” (2020).
- MN Editors. (2022, 22 januari). Comparison Between Gram Stain and Acid Fast. Microbiology Note. Geraadpleegd op 15 maart 2022, van https://microbiologynote.com/comparison-between-gram-stain-and-acid-fast/
- Lakna, B. (2019, 13 februari). What is the Difference Between Gram Stain and Acid Fast? Pediaa.Com. Geraadpleegd op 15 maart 2022, van https://pediaa.com/what-is-the-difference-between-gram-stain-and-acid-fast/

whats the reason of bipolar staining of pasteurella mutocida serotype B:2???why it gets bipolar staining??
Examples of Gram Positive Organisms
Bacillus, Nocardia etc
Examples of Gram Positive Organisms
Escherichia, Helicobcater, etc
you have the same headline for both 😀
also it would be perfect if you could write which bacteria you used for the gram positive/negative image