The Ziehl-Neelsen stain (ZN stain), also called the hot method of AFB staining, is a type of differential bacteriological stain used to identify acid-fast organisms, mainly Mycobacteria.
Acid fast organisms are those which are capable of retaining the primary stain when treated with an acid (fast=holding capacity). Members of the Actinomycetes, genus Nocardia (N. brasiliensis and N. asteroides are opportunistic pathogens) are partially acid-fast. Oocysts of coccidian parasites, such as Cryptosporidium and Isospora, are also acid-fast.
You May also like :
- Auramine-Rhodamine Staining for AFB : Principle, Procedure, Reporting and Limitations
- Lowenstein Jensen (LJ) Medium : Composition, Preparation, Method of Use and Pictures
The ZN staining technique is based on the work of five people. Koch, Ehrlich, Ziehl, Rindfleisch and Neelsen, but it is popularly known as the ‘Ziehl-Neelsen’ method.
- Robert Koch stained tubercle bacilli by immersing in a hot alkaline solution of methylene blue for 24 hours (1882)
- Ehrlich discovered the distinctive property of acid-fastness ie, ability to resist decolourisation with acid (1882). He stained bacillus with basic fuchsin in the presence of aniline oil as mordant and used dilute mineral oil for decolorization.
- Ziehl used carbolic acid as the mordant instead of aniline oil (1882).
- Rindfleisch heated the slide for few minutes instead of putting into a hot solution and reduced the staining time (1882).
- Neelsen combined Basic fuchsin and carbolic acid together and used these as a single solution (1883).
Principle of Ziehl-neelsen Stain
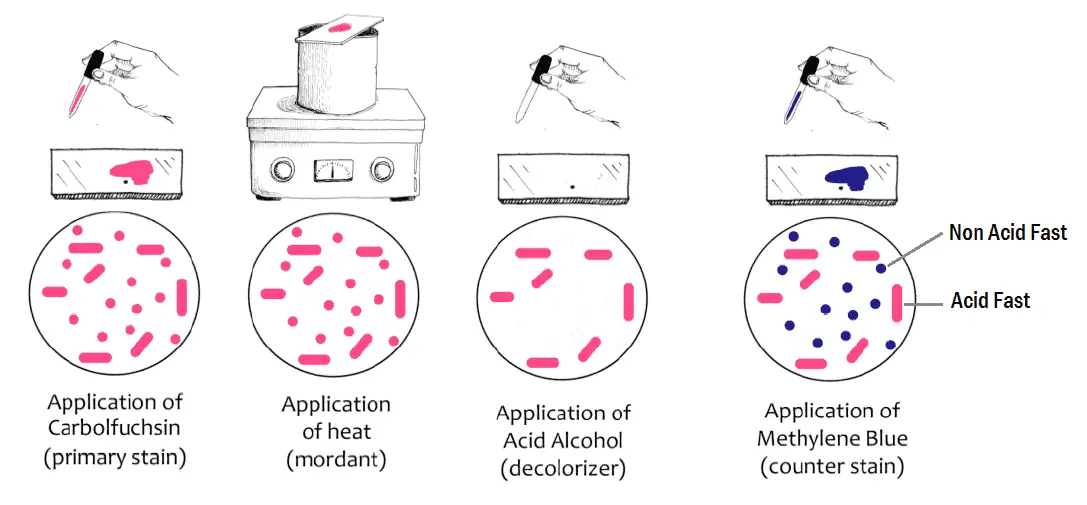
Organisms such as Mycobacteria are extremely difficult to stain by ordinary methods like Gram Stain because of the high lipid content of the cell wall. The phenolic compound carbol fuchsin is used as the primary stain because it is lipid soluble and penetrates the waxy cell wall.
Staining by carbol fuchsin is further enhanced by steam heating the preparation to melt the wax and allow the stain to move into the cell. Acid is used to decolorize nonacid-fast cells; acid-fast cells resist this decolorization. The ability of the bacteria to resist decolorization with acid confers acid -fastness to the bacterium.
Following decolorization, the smear is counterstained with malachite green or methylene blue which stains the background material, providing a contrast colour against which the red AFB can be seen.
Acid alcohol can also be used as decolorizing solution, resistant organisms are referred to as Acid Fast Bacilli (AFB) or Acid Alcohol Fast Bacilli (AAFB).
Requirements
- Primary stain : 0.3% Carbol Fuchsin – Dissolve 50g phenol in 100ml ethanol (90%) or methanol(95%). Dissolve 3g basic fuchsin in the mixture and add distilled water to bring the volume to 1 L.
- Decolorization solution : 25% Sulphuric acid
- Counter stain : 0.3% methylene blue or malachite green.
Procedure of Ziehl-Neelsel stain
- Make a thin smear of the material for study and heat fix by passing the slide 3-4 times through the flame of a Bunsen burner or use a slide warmer at 65-75 C. Do not overheat.
- Place the slide on staining rack and pour carbol fuschin over smear and heat gently underside of the slide by passing a flame under the rack until fumes appear (without boiling!). Do not overheat and allow it to stand for 5 minutes.
- Rinse smears with water until no color appears in the effluent.
- Pour 20% sulphuric acid, wait for one minute and keep on repeating this step until the slide appears light pink in color (15-20 sec).
- Wash well with clean water.
- Cover the smear with methylene blue or malachite green stain for 1–2 minutes.
- Wash off the stain with clean water.
- Wipe the back of the slide clean, and place it in a draining rack for the smear to air-dry (do not blot dry).
- Examine the smear microscopically, using the 100x oil immersion objective.
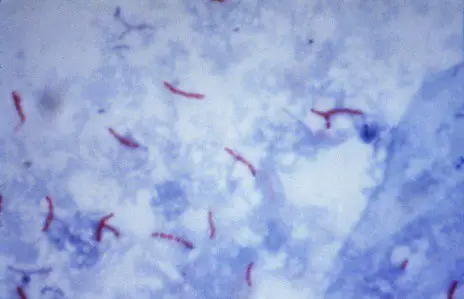
Results and interpretation
- Acid Fast Bacilli : Red, straight or slightly curved rods, occurring singly or in small groups, may appear beaded
- Cells : Green (malachite green) or Blue (methylene blue)
- Background material : Green (malachite green) or Blue (methylene blue)
List of Acid Fast organisms
- Mycobacterium spp: Acid Fast
- Cyst of Cryptosporidium: Acid Fast
- Cyst of Isospora: Acid Fast
- Nocardia spp: Partial Acid Fast
- Rhodococcus spp: Partial Acid Fast
- Legionella micdadei: Partially acid fast in tissue
Reporting the sputum smear
When any definite red bacilli are seen :
Report the smear as ‘AFB POSITIVE’, and give an indication of the number of bacteria present as follows:
| Number of AFB seen (1000X Magnification) | Reported As |
|---|---|
| 0 AFB per 300 Field | AFB Not Seen |
| 1-2 AFB per 300 Fields | Doubtful; repeat with another specimen |
| 1-9 AFB per 100 Fields | 1+ |
| 1-9 AFB per 10 Fields | 2+ |
| 1-9 AFB per Field | 3+ |
| >9 AFB per Field | 4+ |
- When very few AFB are seen:
E.g. when only one or two AFB are seen, request a further specimen to examine. Tap water sometimes contain AFB that resemble tubercle bacilli, and occasionally stained scratches on a slide can be mistaken for AFB. Occasionally AFB can be transferred from one smear to another when the same piece of blotting paper is used to dry several smears. - When no AFB are seen after examining 300 fields: Report the smear as ‘AFB NOT SEEN’. Do not report ‘Negative’ because organisms may be present but not seen in those fields examined. Up to three specimens (one collected as an early morning specimen) may need to be examined to detect M. tuberculosis in sputum.
Modifications of ZIEHL-NEELSEN STAIN
Use of alcohol as secondary decolorizer :
After primary decolorization with sulfuric acid, the smear may be treated with 95% alcohol as secondary decolorizer. M. tuberculosis is both acid fast and alcohol fast, while saprophytic mycobacteria are only acid fast.
Use of acid-alcohol as decolorizer :
Instead of using 20% sulfuric acid, 3% HCl in 95% alcohol may be used. This also differentiates tubercle bacilli from saprophytic mycobacteria. It is especially used in diagnosis of renal tuberculosis.
Modifications in the percentage of sulfuric acid :
- <5% H2SO4 for M. leprae.
- 1% H2SO4 for Actinomyces in tissue.
- 0.5% H2SO4 for cultures of Nocardia.
- 0.25-0.5% H2SO4 for spores and the oocysts of Cryptosporidium and Isospora.

thank u for teaching us but i want to know abou cold techinique how can be performed?
one more technique blotting paper method is available. The procedure time is lesser than other technique. It will take just 4 minutes
* take a blotting paper and cut it slide size
* place it cover the slide
* add few drops carbol fuchsin strong in the blotting paper
* heat below the slide as gentle for 2-3 min
* remove the blotting paper and wash with distilled water
* add 20% H2So4 for 1 min and wash with distilled water
* add Methylene blue for 1 min
* wash with distilled water
* blot with blotting paper examine under oil immersion
A good source of better knowledge.
thanks for the information
Thanks very much for the filter paper method of afb technique that is less time consuming. What is function of the filter in the method?
stunning procedure and simply thanks
Thanks for good teaching.
Good work