The measurement of how light interacts with materials is studied through spectrophotometry.
It is done with the use of a spectrophotometer, a device that measures the intensity of light as a beam of light passes through a sample solution. Light is a versatile element. It can be reflected, scattered, transmitted, and absorbed.
Different materials have the ability to emit light in different ways such as absorption, reemits, or through temperature/incandescence. The measurement of spectrophotometric properties is discussed below.
There are different instruments used to measure different things and one of which is spectrophotometer.
What is a spectrophotometer? It is an optical instrument that measures the intensity of light in relation to the wavelength. It can measure just about anything such as:
- Plastics
- Paper
- Liquid
- Metal
- Fabric
Being a color measurement device, it makes sure that the color is consistent from conception to delivery. As a part of the color control program, designers and brand owners use a spectrophotometer to capture and evaluate color and monitor color accuracy throughout production.

Image 1: The image above shows the typical/basic structure of a spectrophotometer.
Picture Source: encrypted-tbn0.gstatic.com
Why is the spectrophotometer important?
With the use of such a device, the person using a spectrophotometer can easily acquire spectra of shining white light on a given sample thereby measuring the light that is returned from the sample.
The device is used in many different fields, especially in the production facilities and scientific laboratories. In a production facility, a spectrophotometer is used to check for the quality of product such as in clothing and emission of light during the manufacture of LED light, to name a few.
In analytical laboratories, a spectrophotometer is used for the identification and quantifying microscopic samples such as matching colors, kinetics, qualifying gems and minerals, determining the color of paint or ink, and so on. (1, 2, and 3)
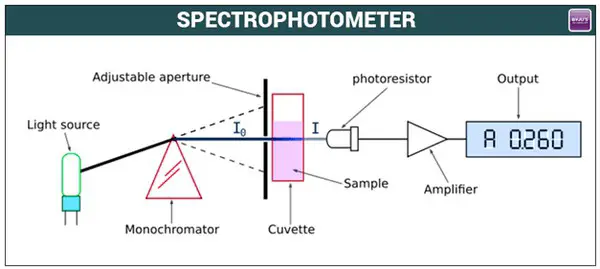
Image 2: The principle of a spectrophotometer as shown in the diagram above.
Picture Source: encrypted-tbn0.gstatic.com
Spectrophotometer principle
A spectrophotometer is a refined version of a colorimeter. In other words, it functions the same way as a colorimeter but with added features. A colorimeter uses a filter which enables a broad range of wave lengths to pass through.
A spectrophotometer a prism or grating is used for the incident beam to split into different wavelengths. The waves of the particular wavelengths can be adjusted to fall on the test solution. (2)

Image 3: The spectrophotometer is calibrated using a potassium dichromate.
Picture Source: encrypted-tbn0.gstatic.com
Spectrophotometer calibration
It is a process by which the scientist or researcher uses a calibration standard to find out the light source’s accuracy. It is vital to make sure that the device functions properly and the correct measurement is obtained. The calibration technique varies according to the make and brand. (3)
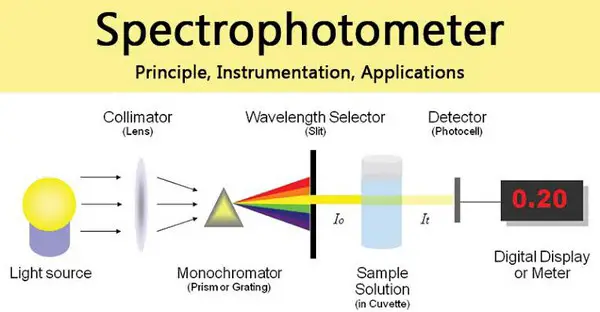
Image 3: The applications of a spectrophotometer as shown in the image.
Picture Source: encrypted-tbn0.gstatic.com
Applications of Spectrophotometer
- It is useful in qualitative analysis, especially when identifying classes of compounds in both biological and pure state.
- The spectrophotometer is essential in quantitative analysis of biochemistry practical such as in determining the unknown concentration of a given species through absorption spectrometry. A perfect example is the nucleic acid in a protein.
- Enzyme assay is the primary use of spectrophotometry.
- Identifying the molecular weight of a particular sample such as amine picrates, ketone compounds, aldehyde, and sugar, to name a few. (4, 5)
Other primary applications of spectrophotometer include the following:
- Identifying impurities.
- Detecting the concentration of substances.
- Organic compounds’ structure elucidation.
- Identifying the characteristics of a protein.
- Identifying the dissolved oxygen content in a body of water.
- Analysis of respiratory gas in hospitals.
- Functional group detection.
- Determining molecular weight in a particular compound.
- Identifying classes of compounds. (4, 5, and 6)
How does a spectrophotometer work?
- A sample of the subject being studies is placed in the spectrophotometer.
- The light source shines the sample and the monochromator splits the light into each color/individual wavelength.
- The light’s wavelength hits the subject that is held in cuvette – a tiny container. Careful handling should be observed as even the slightest fingerprint can alter the result
- The light that passes through the sample is read and interpreted as seen on the output screen. (5, 6)
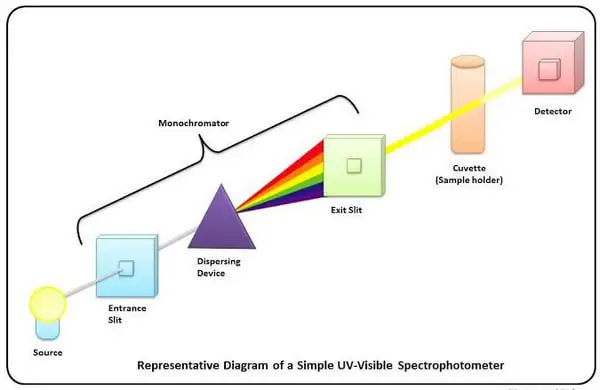
Image 4: These are the basic components of a spectrophotometer.
Picture Source: encrypted-tbn0.gstatic.com
What are the three main components of a spectrophotometer?
The main components of a spectrophotometer are the light source, a device that separates the light into component wavelengths, a sample holder and a detector.

Image 5: It is an example of a visible light spectrophotometer.
Picture Source: encrypted-tbn0.gstatic.com

Image 6: The image above is an example of a UV/visible spectrophotometer.
Picture Source: encrypted-tbn0.gstatic.com

Image 7: An example of a near infrared spectrophotometer.
Picture Source: encrypted-tbn0.gstatic.com
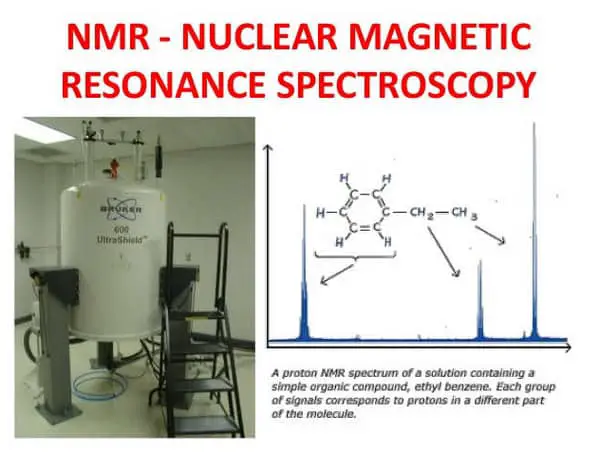
Image 8: A nuclear magnetic resonance spectroscopy.
Picture Source: encrypted-tbn0.gstatic.com

Image 9: An atomic absorption spectrophotometer.
Picture Source: encrypted-tbn0.gstatic.com


Image 9: This is how a mercury analyzer in a laboratory setting looks like.
Picture Source: encrypted-tbn0.gstatic.com
What are the different types of spectrophotometer?
- Visible light spectrophotometer – This type of spectrophotometer uses a visible light from a tungsten lamp. It is typically used for routine laboratory work, specifically the portable and bench-top spectrophotometer models.
- UV/Visible spectrophotometer – A visible light spectrophotometer is turned into a UV-visible unit with the aid of a second lamp. It can measure up to 1100 nm. It comes with a wide array of features like scanning function, user interface, integral printer, and multiple cell setting.
- Near-infrared spectrophotometer – It functions the same way as the UV spectrophotometer, but the difference is it measures the response of a sample when exposed to infrared light. It provides a non-invasive analysis and a quantitative finding with only a minimal sample preparation. Near-infrared spectrophotometer is helpful in monitoring highly absorbing solids. It also provides essential information like fat, protein, fiber, and starch content.
- Nuclear Magnetic Resonance spectroscopy – it is a powerful tool used to determine the structure of organic compounds. It provides structural detail of the entire molecule as well as dynamic information of organic reactions.
- Atomic absorption spectrophotometer – a flame evaporates water from the sample causing it to dissociate into ions. The dissociation leads to changes in the intensity of light as seen by the detector. Hence, help in finding out the concentration of the sample. Atomic absorption spectrophotometer’s high precision analysis is useful in toxicology, environmental testing, and quality control laboratories.
- Mercury spectrophotometer/analyzer – It instantly measures the trace amount of mercury in water.
- Fluorometers – It measures the fluorescence release once the object being studied is exposed to a single wavelength of light. (5, 6, and 7)

Image 11: A portable and a bench-top spectrophotometer.
Picture Source: encrypted-tbn0.gstatic.com
What are the different categories of spectrophotometer?
- Portable spectrophotometers – As the name suggests, this type of spectrophotometer is something you can carry with you any time and anywhere. You can easily put it in your pocket and take out whenever you need to.
- Bench-top spectrophotometer – It is the ideal choice for laboratory-based procedures, especially in subjects that need the highest level of control and precision. (7, 8)
Difference between a spectrometer and a spectrophotometer
A spectrometer is used by scientists to gather details of a substance based on the light it projects, be it visible, ultraviolet, or infrared. It is applicable in different fields of science. In astronomy, astronomers used spectrometers to check the object’s temperature while in space.
They also use spectrometer to measure the speed it travels and estimate the weight of the object. In a scientific study, scientists use spectrometer to find out the composition of things on earth and/or in space including the elemental components. In a laboratory setting, spectrometers can identify toxins in the bloodstream, contaminants, and diseases.
On the other hand, the spectrophotometer is a tool designed to measure the intensity of electromagnetic radiation at different wavelengths. It measures the absorbency of the wavelength in a given solution, transparency or transmittance of solids, and reflectance of solutions.
In an electromagnetic radiation spectrum, the spectrophotometer can assess the diffusivity of the light range, especially those with various calibrations and controls. Spectrophotometers have two basic classifications too – double beam and the basic.
The double beam compares the intensity of light between the reference light path and the substance being measured. The basic measures the relative light intensity of the beam before and after introducing the sample. (7, 8, and 9)
Differences between spectrometer and spectrophotometer
- A spectrometer is a component of spectrophotometer used to measure different kinds of items.
- A spectrophotometer is a complete system consists of a light source that gathers light that interacted with the subject and the spectrometer for measurement.
- They differ in terms of usage. When using the spectrometer, you have to wait for it to heat up after turning it on. the subject to be studied is loaded and calibrated to determine the spectrum. Only then will the wavelengths be measured and analyzed. The subject being studied is loaded and the light passes through the machine. Readings are made according to the reflected colors and information.
- When it comes to using a spectrophotometer, you have to be very careful ensuring that no dirt or fingerprint is in the machine. A solute is added and the spectrophotometer is set to the desired wavelength. The empty cuvette is inserted making sure that the arrow is aligned. The spectrophotometer is calibrated by pressing “set zero” button. Place the solution to know the absorbency. (8, 9, and 10)
Difference between UV (ultraviolet spectroscopy) and visible spectrophotometry
The ultraviolet spectroscopy is an absorption type UV spectroscopy, which is the visible part of the electromagnetic spectrum. The non-binding electron microscope can absorb energy in either visible light or ultraviolet rays to excite the electrons to achieve a high molecular orbital.
This has something to do with the absorption ability as easily excited electrons have a longer wavelength of light to absorb. With reference to the quantum mechanical selection rule, the molecule is in a singlet excited state.
The very same method is used in fluorescence spectroscopy. The fluorescence is the relaxation phase in which a molecule in the excited phase can relax back to the ground state.

Image 12: A double-beam spectrophotometer.
Picture Source: encrypted-tbn0.gstatic.com

Image 13: A split beam spectrophotometer.
Picture Source: encrypted-tbn0.gstatic.com
Difference between a double-beam spectrophotometer and a split beam spectrophotometer?
As mentioned above, a spectrophotometer measures the light’s wavelength distribution. In a double beam spectrophotometer, a real-time referencing is allowed using a separate reference position in the spectrophotometer.
In a split beam spectrophotometer, the machine uses a beam splitter rather than a copper to send light along the sample and blank paths to two separate detectors. Both measurements in the blank and sample can be made.
Both types are useful in various fields, especially in applications needing stability, high speed, and flexibility. The measurements achieved are more reproducible making them a must-have instrument in both industrial and laboratory setting. If you are going to purchase a spectrophotometer, be it a split or double-beam, you have to consider the following:
- Long lamp life
- Method of storage
- On-board software
- Sample changers
- Self-test diagnostics for validation of LP instrument performance
- Service contracts
- Extended warranties
- Accessories for specific applications like tube adapters, temperature control, and automatic sippers. (1, 3, and 5)
Difference between a colorimeter and spectrophotometer
Both are used to measure color-absorbing properties of a particular substance. In chemistry, both are used to measure the solution’s color absorption. In colorimeter, the specific color absorbance is measured.
In a spectrophotometer, the reflectance of transmittance is measured as a function of the wavelength. Colorimeters have a set of colored filter or LED bulb that can emit a particular color of light. When using a colorimeter, you have to choose the appropriate color and a cuvette with a solution is placed inside the colorimeter.
The absorbance for the chosen color is revealed. One thing to keep in mind – a solution of a particular color absorbs its own color the least.
Differences between a colorimeter and a spectrophotometer
- They differ greatly in functions. A colorimeter is designed to measure the absorption ability of a particular color in a given sample. On the other hand, the spectrophotometer measures the sample’s transmittance or reflectance of color as a function of wavelength.
- They differ in range. A colorimeter only works with light in the visible part of the electromagnetic spectrum. On the other hand, the spectrophotometer has the ability to work with both infrared and ultraviolet light and visible light.
- They differ in cost. because of the complexity of functions, spectrophotometer is more expensive than the colorimeter. (4, 7, 9, and 10)
References
- http://www.microspectra.com/support/learn/what-is-a-spectrophotometer
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/spectrophotometers
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/Reaction_Rates/Experimental_Determination_of_Kinetcs/Spectrophotometry
- https://en.wikipedia.org/wiki/Spectrophotometry
- https://www.jove.com/science-education/5038/introduction-to-the-spectrophotometer
- https://study.com/academy/lesson/spectrophotometer-definition-uses-parts.html
- https://www.carolina.com/teacher-resources/Interactive/what-goes-on-inside-a-spectrophotometer/tr41103.tr
- https://www.xrite.com/blog/spectrometer-vs-spectrophotometer
- https://www.hitachi-hightech.com/global/product_list/?ld=sms1&md=sms1-3
- https://www.ruf.rice.edu/~bioslabs/methods/protein/spectrophotometer.html
