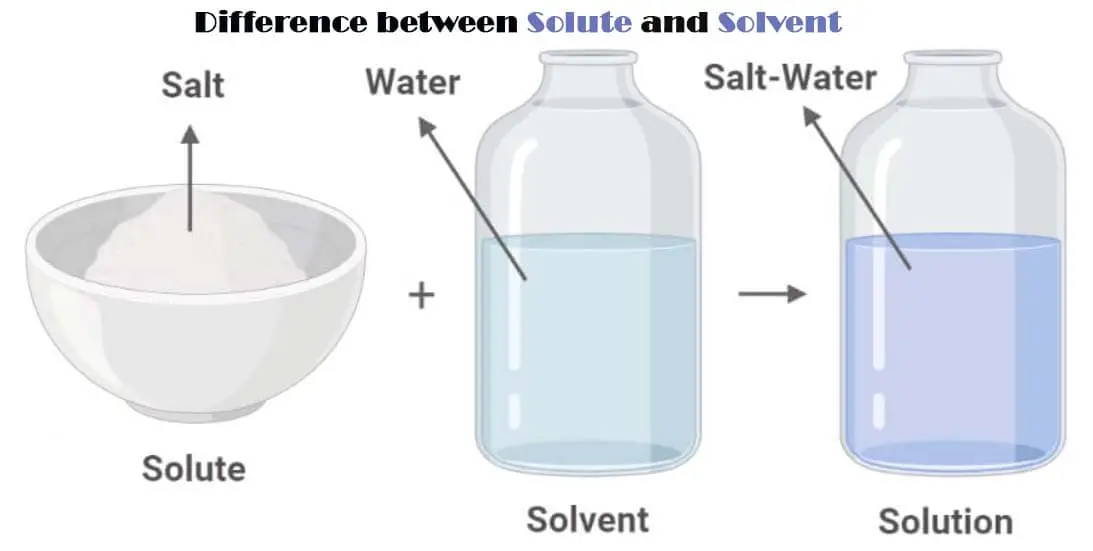
A solution is formed when one particular substance dissolves into another. It is a homogenous mixture made up of a solute and a solvent.
In a nutshell, the substance being dissolved is called a solute while the dissolving medium is called the solvent.
The ability of the solute to dissolve in a particular solvent is called solubility. While solute and solvent are necessary to form a solution, you need to know that there’s a difference between the two.
To know more about solute and solvent including their similarities and differences, keep on reading below. (1, 2, and 3)
Solute
It pertains to the substance being added to the solvent. It can be in the form of solid, liquid, or gas. Its ability to dissolve in a given solvent is referred to as solubility.
In a solution, the solute makes up only a small quantity when compared with the solvent. Examples of solutes are the following:
- Sugar in a tea
- Salt in seawater
- Protons in the cytosol (2, 3, and 4)
Solvent
It pertains to substances that have the ability to dissolve solute particles. The majority of solvents are liquid and a few are solid and gas. What the solvent does is it breaks down large solute particles into tiny pieces. Examples of solvents are the following:
- Water
- Alcohol
- Esters
- Hydrocarbons (4, 5)
Refer to the table below for the differences between solvent and solute.
| Key differences/point of comparison | Solute | Solvent |
|---|---|---|
| Meaning/definition | It is the substance added to the solvent. It is the one being dissolved to form a solution (5) | It is a substance used to dissolve a solute thereby forming a solution. (4) |
| Division or category | Solute has no division or category. (4) | The solvent can be classified as polar or non-polar.
|
| Physical state | It is available in three different states namely solid, liquid, and gas. (4, 5) | The majority of solvents are liquid, but you can also find solvent in the gas state. (5, 6) |
| Phase | It is the solution’s dispersed phase. | It is the solution’s medium phase responsible for dispersing the particles of solute. |
| Quantity | The solute is usually lesser than the solvent. (4) | The solvent is always more than the quantity of solute. |
| Solution state | It can or can be in the solute state. | The solution is most likely the same as the state of the solvent. |
| Solubility | It all depends on the property of the solute, specifically the size of the molecules and the surface area. (5, 7, and 8) | It has something to do with the property of the solvent such as its polarity. (9) |
| Boiling point | When compared with the solvent, the solute has a higher boiling point. | When compared with the solute, the solvent has a lower boiling point. |
| Transfer of heat | The heat of the solution is transferred to the solute. | It is the solvent that transfers heat to the solution. (5) |
| Dependability | The solubility has something to do with the properties of the solute. (3, 4) | The properties of the solvent can greatly affect solubility. |
| Examples |
|
|
Below is the summary of the key differences between solvent and solute:
- Solute is the substance that the solvent dissolves in a given solution. On the other hand, the solvent pertains to the substance that is used to dissolve the solute.
- In terms of quantity, the solvent is always greater than the solute.
- Solute can be in the form of solid, liquid, or gas. However, most solvents are in the form of liquid. In a rare solution, the solvent can be in the state of solid or gas.
- When it comes to the boiling point, the solute always has a higher boiling point than that of the solvent.
- Property-wise, both solute, and solvent are interdependent with each other.
- The solubility has something to do with the solute’s property such as the surface area and chemical structure. In the solvent, the solubility greatly depends on the property of the solvent particularly the temperature and polarity.
- In a given solution, the heat is transferred to the solute while the heat is transferred from the solvent.
- The amount of solute in a given solution is measured through its concentration, which is dependent on the ratio of the solute and the solution’s total volume.
- The majority of solvents are classified into polar and non-polar. There is one special category though and it is called amalgams where mercury formed a specific solvent.
- The universal solvent is water, which is also classified as a polar compound. It has the ability to dissolve a large number of solute particles.
- Both the solute and solvent in a given solution exist in a single-phase, which forms solute-solvent complexes known as solvates.
- Solvent makes up most of the volume of the solution. It forms the solution’s medium and its quantity is huge enough to dissolve a particular solute. The amount of solute that the solvent can disperse is dependent on the medium’s temperature. (3, 6, 8, 9, and 10)
A solution won’t be called a solution if it is not made up of solute and solvent. However, of the two, the solvent makes up most of the solution making the solute a minor component.
Although there are many components that make solute and solvent different from each other, the major difference is that the solute is the substance being dissolved while the solvent is the substance used to dissolve the solute.
References
- https://microbenotes.com/solute-vs-solvent/
- https://biodifferences.com/difference-between-solute-and-solvent.html
- https://www.toppr.com/guides/biology/difference-between/solute-and-solvent/
- https://vivadifferences.com/solute-vs-solvent/
- https://medium.com/@dikshabhardwaj283/what-is-the-difference-between-solute-and-solvent-ceb24db263bc
- https://pediaa.com/difference-between-solvent-and-solute/
- https://dashamlav.com/solute-vs-solvent-differences-examples/
- https://byjus.com/chemistry/solute/
- https://byjus.com/chemistry/difference-between-mixture-and-solution/
- https://biodifferences.net/difference-between-solute-and-solvent/
