Oxidase test is a procedure used to determine the existence of cytochrome c oxidase enzyme. What the system does is it helps the movement of electrons between redox dye- tetramethyl-p-phenylene-diamine and the bacteria’ electron donors.
It helps identify bacteria capable of producing cytochrome c oxidase; an enzyme present in the bacterial electron transport chain. It is called oxidase test because if the enzyme is present, the enzyme will not lead to oxidation of the reagent thereby producing purple color end product (indophenols).
There is no oxidation if no enzyme is found. Hence, the color of the reagent remains the same. (1, 2, and 3)

Image 1: The image shows an aerobic bacteria set for oxidase test.
Picture Source: ytimg.com
What to keep in mind?
Bacteria that test positive for oxidase test are aerobic. They can make use of oxygen in respiration. On the other hand, bacteria that test negative for oxidase test can be aerobic, anaerobic, or facultative.
This means that such organisms do not have the enzyme cytochrome c oxidase which is needed for the reagent to oxidize. (2, 3)
What are the requirements?
- Moist filter paper with substrate
- Paper disk (commercially prepared)
- Wire made from wood or platinum
- Reagents such as:
- Kovacs oxidase reagent
- Gordon and McLeod’s Reagent
- Gaby and Hadley (indophenol oxidase) Reagent: (2, 3, 4, and 5)
What is the purpose of oxidase test?
- It helps determine if the organism has a cytochrome oxidase enzyme.
- It helps in differentiating certain strains of bacteria like Moraxella, Neisseria, Pasteurella species, and Campylobacter.
- It helps in differentiating pseudomonads from other related species. (4, 6)
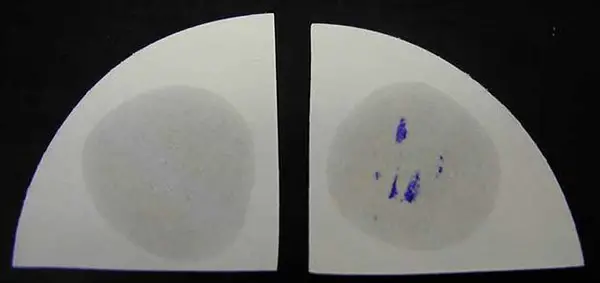
Image 2: Wet filter method oxidase test.
Picture Source: microbiologyinfo.com

Image 3: Dry filter method oxidase test.
Picture Source: microbiologyinfo.com

Image 4: Direct plate method oxidase test.
Picture Source: emedicalhub.com
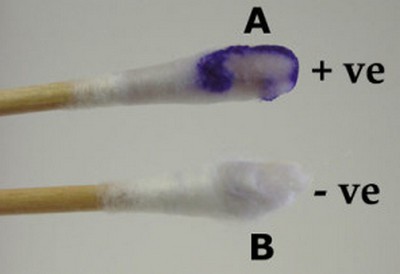
Image 5: Swab method oxidase test.
Picture Source: microbiologyinfo.com

Image 6: Test tube method oxidase test.
Picture Source: emedicalhub.com
Procedures
Oxidase test can be done using various methods. We will discuss the commonly used methods below.
- Dry filter paper – This is the most convenient method. Procedures are as follows:
- Soak the filter paper (Whatman’s # 1) in a 1% tertramethyl-p-phenylene-diamine dihydrochloride solution.
- Drain for about half a minute.
- Freeze dry the filter paper and store in a bottle; preferably the dark one and with a cap secured by screws.
- If used, it is a must to remove the strip, use distilled water to moisten the strip and put in the Petri dish.
- Using the loop (platinum), pick the colony and smear over the moist section.
- Wet Filter Paper – It is called the wet filter paper method because the filter is soaked in a 1% solution of the reagent. Using a loop (made from platinum material), rub the culture on the filter paper.
- Direct Plate – Add a reagent; ideally two to three drops to the suspected colonies on the agar plate. Just add enough reagent as putting too much reagent can alter the result. Watch out for any changes in color in a span of 10 seconds. This type of oxidase test should be done on the agar plate (non-selective).
- Swab method – the swab should be dip into the reagent and should be in contact with the suspected isolated colony. Watch out for any changes in color in 10 seconds.
- Test tube – Fresh bacteria culture is grown in a nutrient broth (4.5 ml). Growing should be done within 18 to 24 hours. Add Gaby and Hadley reagent (0.2ml). Shake the test tube to make sure the mixture is thoroughly mixed and the culture has thorough oxygenation. Watch out for any changes in color. (4, 7, 8, 9, and 10)
Result Interpretation
- Positive – The result is positive if you notice an intense deep-purple hue within 10 seconds.
- Delayed positive – If the deep-purple hue appeared within 60 seconds.
- Negative – The result is negative if the color didn’t change at all or if it changes after 60 seconds. (1, 3, and 8)
Note when using the reagents
- Kovac’s oxidase reagent – When using this reagent, the organisms being tested would turn out positive if the color of the medium changes to dark purple within 10 seconds. A delayed positive test is noted if the color turns to dark purple within 60 to 90 seconds. On the other hand, the test is negative is no changes in color took place after two minutes.
- Gordon and McLeod reagent – The organisms being tested are positive for oxidase test if the color turns red within 10 to 30 minutes or if it changes to black within 60 minutes. If there are no changes after that given timeframe, then the test is negative. (2, 4, 9, and 10)
Examples of bacteria that test positive for oxidase test
- Neisseria
- Pseudomonas
- Vibrio cholerae
- Campylobacter spp
- Aeromonas spp
- Helicobacter spp/ Haemophilus spp
- Alcaligens
- Brucella
- Moraxella
- Pasteurella
- Legionella pneumophila (4, 5)
Examples of organisms that test negative for oxidase test
- Enterobacteriaceae family
Are there any limitations?
- When performing the test, there is a possibility that the reagent can auto-oxidize. Hence, it is of utmost importance to use only fresh reagents. For the reagent to be considered fresh, it should no longer than one week.
- It is important to check the colonies on the media that do not contain excessive sugar like nutrient agar and tryptic soy agar. A high concentration of glucose in the media leads to a false positive result.
- The result may be aberrant if the microorganism being tested is grown on the media containing dyes.
- Perform sub-culturing before adding the reagent on the active culture as some reagents can kill the microorganisms.
- Oxidase test is helpful in identifying Neisseria as well as identifying and differentiating gram-negative bacilli. To find out the gram reaction and morphology of the organism being tested, it should be checked using gram stain.
- Always use platinum loops. Using loops that contain iron may lead to a false-positive result.
- A false negative result is possible if the reagent or strips used are less sensitive.
- Make sure that the colonies used for oxidase test are 18 to 24 hours old. If you are going to use older colonies, it will lead to a weak reaction. (3, 9, and 10)
References
- https://en.wikipedia.org/wiki/Oxidase_test
- https://microbeonline.com/oxidase-test-principle-procedure-and-oxidase-positive-organisms/
- https://microbiologyinfo.com/oxidase-test-principle-uses-procedure-types-result-interpretation-examples-and-limitations/
- http://www.asmscience.org/content/education/protocol/protocol.3229
- http://delrio.dcccd.edu/jreynolds/microbiology/2421/lab_manual/oxidase.pdf
- http://www.vumicro.com/vumie/help/VUMICRO/Oxidase_Test.htm
- http://spot.pcc.edu/~jvolpe/b/bi234/lab/differentialTests/oxidase_test.htm
- https://www.microbiologyresearch.org/docserver/fulltext/ijsem/26/2/ijs-26-2-127.pdf?expires=1550857562&id=id&accname=guest&checksum=30FBEEC026731A26657033D0F6D09934
- http://www.biologypractical.com/oxidase-test-principle-procedure-result-interpretation-precautions/
- https://microbenotes.com/oxidase-test-principle-procedure-and-results/
