What is a Nitrate Reduction Test?
It is a procedure that differentiates the members of Enterobacteriaceae based on the ability to produce nitrate reductase enzyme which in turn hydrolyze nitrate to nitrite and will be eventually degraded to different nitrogen products such as nitrous oxide, nitrogen oxide, and ammonia.
Other uses of nitrate reduction test are as follows:
- It helps in differentiating Mycobacterium species.
- Nitrate reduction test can help identify Neisseria species and separate them from other species like Moraxella and Kingella.
- It helps differentiate N. gonorrhoeae from K. denitrificans.
- It helps in identifying Corynebacterium species. (1, 2, and 3)

Picture 1: Four test tubes; the first one is uninoculated, the second test tube does not have a zinc, the third has a zinc, and the last test tube turns red after adding a zinc powder.
Image Source: germsandworms.files.wordpress.com
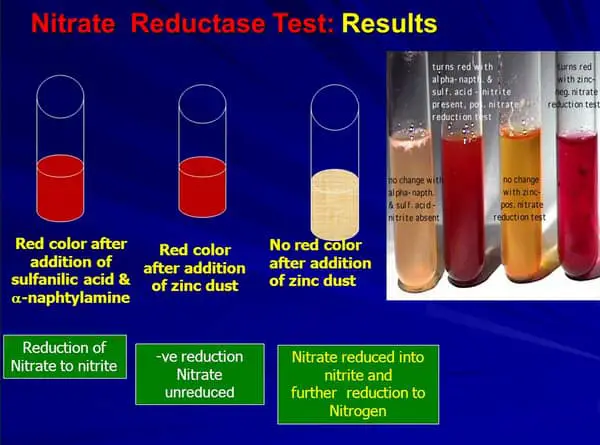
Picture 2: The image shows the result of the nitrate reduction test.
Image Source: slideplayer.com
What is the objective of the nitrate reduction test?
The primary objective of the nitrate reduction test is to check the organism’s ability to produce nitrate reductase enzyme.
By doing the test, it will be easy to differentiate members of Enterobacteriaceae that have the ability to produce nitrate reductase from Gram-negative organisms that do not produce the said enzyme. (3, 4)
Nitrate reduction test principle
Under the anaerobic environment, some microorganisms have the ability to reduce nitrate salts like KNO3 to a nitrite salt. The process is called anaerobic respiration wherein the molecule of nitrate serves as an electron acceptor.
The nitrate reacts with reagents A and B causing red color because of the formation of Azo dye. In some instances, some microorganisms can further reduce nitrites to free nitrogen and ammonia
The incubation period
An inoculum of an organism being tested is incubated in a nitrate broth. It should be incubated for four hours and the broth is checked for the reduction of nitrate to nitrite. It is done by adding sulfanilic acid reagent and α- naphthylamine.
If the microorganism being tested has reduced nitrate to nitrite, you will notice the formation of nitrous acid. If you add sulfanilic acid, it will reach and produce diazotized sulfanilic acid, which will then react to α-naphthylamine forming a color red compound.
Changes in color:
The organism being tested for nitrate reduction is positive if the medium turns red after adding nitrate reagents. If there are no changes in the color of the medium after adding the reagent, it means that the organism being tested does not have the ability to reduce nitrate. It can also mean that the organism has denitrified nitrate or nitrite so as to create ammonia or molecular nitrogen.
If no changes in the color of the medium, you need to proceed with the next step which is adding a small amount of powdered zinc. If the medium turns red, it indicates that there is unreduced nitrate indicating that the result is a true negative.
Keep in mind that if the medium does not turn red after adding zinc powder, it means that the nitrate reduction test result is positive complete. (4, 5, 6, and 7)
Reasons why the culture failed to produce a red color:
- The organism being tested has failed to reduce nitrates.
- The organism being tested has a potent nitrate reductase leading to further reduction of nitrate to ammonia or molecular N2.
Note:
Further testing is needed by simply adding a small amount of zinc powder to the culture. What the zinc powder does is it reduces nitrates to nitrites.
Watch for changes in color. If the color changes to red after adding zinc powder, it means that nitrate was not reduced. (7, 8, and 9)
What are the materials needed for the nitrate reduction test?
- Media specifically nitrate broth with inverted Durhams tube
- Reagents
- Alpha napthylamine reagent
- Sulphalinic acid reagent
- Zinc powder
- Burner
- Inoculating loop
- Dropper
- Organism being tested
- Incubator (10)
How is a nitrate reduction test performed?
- The first step is to inoculate nitrate broth with a growth of organism being tested using a sterile method.
- Incubate.
- Add a drop of sulfanilic acid and a drop of alpha naphthylamine reagent to the broth.
- If the color changes to red, it means that the organism being tested is positive for a nitrate reduction test. (3, 6, and 7)
Additional step:
If the culture does not turn red after adding the necessary reagent, then you need to perform the next step, which is adding zinc powder. Carefully check for changes in color. If the color changes to red, it means that the organism being tested is negative for nitrate reduction test. (2, 5)

Picture 3: Two test tubes; one is nitrate reduction test positive and the other is nitrate reduction test negative.
Image Source: amazonaws.com
Interpreting results:
- Negative – The nitrate reduction test is negative if the color of the medium didn’t change. It means that nitrite is absent. However, another step should be performed to confirm if the result is a true negative. Why? Because some organisms have the ability to further reduce the nitrate to nitrite to another molecule. Therefore, you need to add a small amount of zinc powder to the broth. What the zinc will do is it will catalyze the reduction of nitrate to nitrite. If the color of the medium changes to red, it means that the result is a true negative. If no color change happens after adding zinc powder, it means that the result is positive.
- Positive – A nitrate reduction test is positive if the color changes to cherry red after adding the reagent. If the additional step was taken such as adding a zinc powder, the result is positive if the color of the medium does not turn to red after adding zinc powder. (3, 6, and 8)
Safety measures
Reagents Alpha napthylamine and Sulphalinic acid are poisonous and can be extremely harmful and fatal when swallowed. These reagents should be handled well and should not be in contact with the skin, eyes, and respiratory tract as they are highly corrosive.
If the reagents get in contact with your eyes, you should immediately rinse with water and ask for medical help. On the other hand, zinc powder is extremely flammable once it gets in contact with water.
When storing zinc powder, you have to make sure it is stored in a tightly closed and dry container. In case of fire, do not use water. You can extinguish the fire using sand or carbon dioxide. (1, 5, and 9)
References
- https://catalog.hardydiagnostics.com/cp_prod/content/hugo/nitratereagent.htm
- https://microbeonline.com/nitrate-reduction-test-principle-procedure-results/
- https://www.cdc.gov/std/gonorrhea/lab/tests/nitrate.htm
- http://www.biologypractical.com/nitrate-reduction-test-objective-principle-procedure-and-result/
- https://microbenotes.com/nitrate-reduction-test-objectives-principle-procedure-and-results/
- https://catalog.hardydiagnostics.com/cp_prod/content/hugo/nitratereagent.htm
- https://en.wikipedia.org/wiki/Nitrate_reductase_test
- https://www.sigmaaldrich.com/catalog/product/sial/73426?lang=en®ion=PH
- http://www.asmscience.org/content/education/protocol/protocol.3660
- http://www.vumicro.com/vumie/help/VUMICRO/Nitrate_Reductase_Test.htm
