High-performance liquid chromatography, abbreviated as HPLC, is a chromatographic technique of great versatility and analytic power used in many aspects of drug manufacturing and research.
It separates or identifies mixtures of substances into their components based on their molecular structure and composition.
The other name for high-performance liquid chromatography is high-pressure liquid chromatography.
Introduction
High-performance liquid chromatography (HPLC) is the most widely used separation technique. It can be very sensitive, specific, and precise.
- It is a particular form of column chromatography used in biochemistry and analysis to separate, identify, and quantify the active compounds in a mixture.
- In HPLC, a column holds packing material (stationary phase), a pump moves the mobile phase(s) through the column, and a detector shows the retention times of the molecules.
- Retention time is variable and mainly depends on the interactions between the stationary phase, the molecules being analyzed, and the solvent(s) used.
- A small volume of sample to be analyzed is introduced to the mobile phase stream and is retarded by specific chemical or physical interactions with the stationary phase.
- The amount of retardation mainly depends on the nature of the analyte and the composition of both stationary and mobile phases.
- The most common solvents used in high-performance liquid chromatography (HPLC) are methanol and acetonitrile.
Brief history
Michael Tswett (1872-1920) is credited as the father of chromatography due to his demonstration of liquid chromatography. In 1903, he separated the green-leaf pigments into bands of colors. After that, in 1937-38, thin-layer chromatography (TLC) was used. The next significant advancement was the use of paper chromatography in the mid-1940s.
Thin-layer chromatography (TLC) advanced slowly during the next few years, but Egon Stahl made significant development in 1956. Egon Stahl standardized the preparation of the sorbents used to make the plates. High-pressure liquid chromatography (HPLC) was later developed in the 1970s.
The term high-performance liquid chromatography (HPLC) was introduced in the 1970s to distinguish the modern high-performance technique from classical low-pressure column chromatography, developed in the 1930s.
HPLC Principle
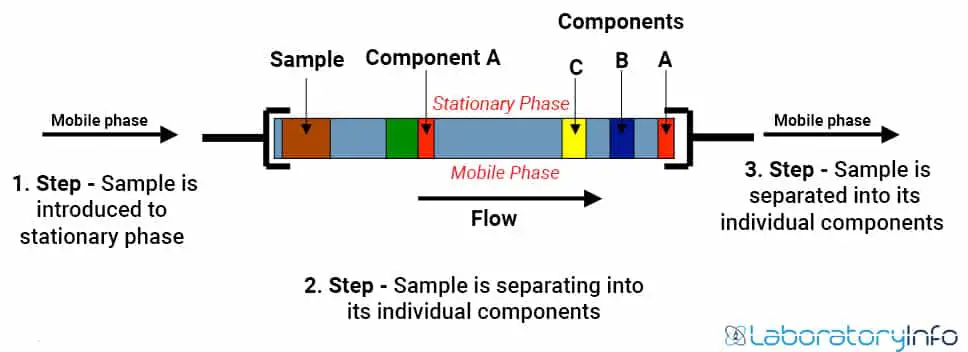
High-performance liquid chromatography (HPLC) involves the injection of a small volume of liquid sample into a tube packed with tiny particles (3 to 5 microns (µm) in diameter called the stationary phase) where individual components of the sample are moved down the packed tube with a liquid (mobile phase) forced through the column by high pressure delivered through a pump.
The column packing is used to separate the components from one another. It involves various chemical and/or physical interactions between their molecules and the packing particles.
The separated components are then detected at the exit of the column by a detector that measures their amount. Output from this detector is called a “liquid chromatogram.”
Advantages over low-pressure column liquid chromatography
There are many advantages of High-performance liquid chromatography (HPLC) over traditional low-pressure column liquid chromatography.
- Greater sensitivity (various detectors can be employed)
- Improved resolution
- Speed
- Easy sample recovery (less eluent volume to remove)
- A wide variety of stationary phases
Branches of HPLC
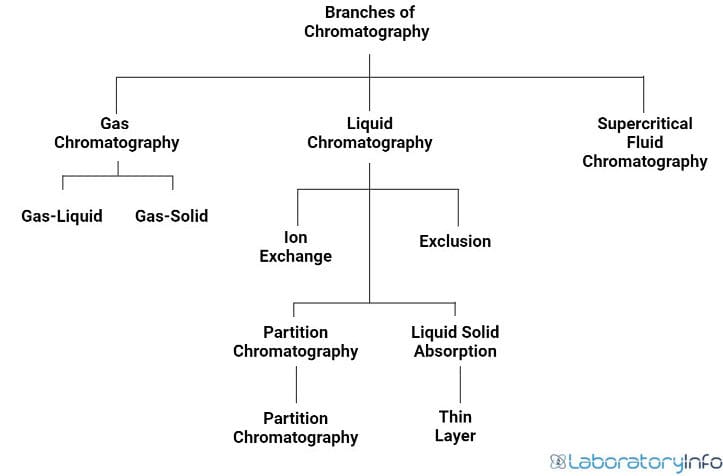
Chromatography is divided into gas, liquid, and supercritical fluid techniques. Gas chromatography is further divided into gas-liquid and gas-solid techniques.
Liquid chromatography is divided into a relatively large collection of techniques like thin layer chromatography. Pressurized liquid chromatography can be divided into ion exchange, exclusion, partition, and liquid-solid chromatography.
Types of HPLC
The following variants of HPLC depend upon the phase system (stationary) in the process.
1. Normal Phase HPLC
- They are also known as normal-phase or absorption chromatography. This method separates analytes based on polarity.
- It has a polar stationary phase and a non-polar mobile phase.
- Therefore, the stationary phase is usually silica, and typical mobile phases are hexane, methylene chloride, chloroform, diethyl ether, and mixtures.
- The technique is used for water-sensitive compounds, geometric isomers, cis-trans isomers, class separations, and chiral compounds.
2. Reverse Phase HPLC
- The stationary phase is nonpolar (hydrophobic), while the mobile phase is an aqueous, moderate polar.
- It works on the principle of hydrophobic interactions; hence the more nonpolar the material is, the longer it will be retained.
- This technique is used for non-polar, polar, ionizable, and ionic molecules.
3. Size-exclusion HPLC
- It is also known as gel permeation chromatography or gel filtration chromatography.
- The column is filled with a material having precisely controlled pore sizes, and the particles are separated according to their molecular size.
- Larger molecules are rapidly washed through the column; smaller molecules penetrate the porous packing particles and elute later.
- Size-exclusion chromatography is also helpful in determining the tertiary and quaternary structure of proteins and amino acids.
- It is also used for the determination of the molecular weight of polysaccharides.
4. Ion-Exchange HPLC
- In this type of chromatography, retention is based on the attraction between solute ions and charged sites bound to the stationary phase.
- Same charged ions are excluded.
- This technique is used in purifying water, Ligand and Ion-exchange chromatography of proteins, high-pH anion-exchange chromatography of carbohydrates and oligosaccharides, etc.
5. Bio-affinity HPLC
- In this type of chromatography, separation is based on the reversible interaction of proteins with ligands.
Instrumentation of HPLC
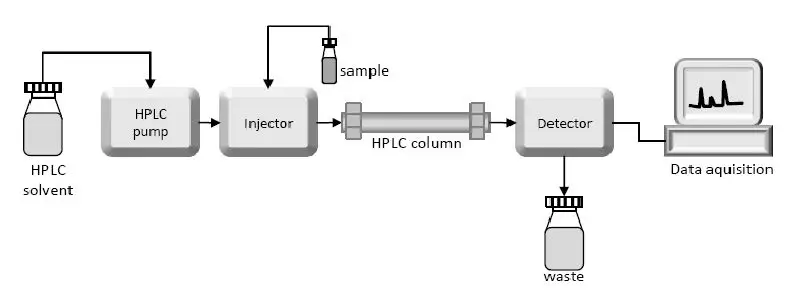
The heart of the system is the column where separation occurs.
1. Solvent Reservoir
- Mobile phase contents are contained in a glass reservoir.
- The mobile phase, or solvent, in HPLC, is usually a mixture of polar and non-polar liquid components whose respective concentrations are varied depending on the composition of the sample.
2. Pump
- A pump aspirates the mobile phase from the solvent reservoir and forces it through the system’s column and detecter.
- Depending on several factors, including column dimensions, the particle size of the stationary phase, the flow rate and composition of the mobile phase, operating pressures of up to 42000 kPa (about 6000 psi) can be generated.
3. Sample Injector
- The injector can be a single injection or an automated injection system.
- An injector for an HPLC system should provide an injection of the liquid sample within the range of 0.1-100 mL of volume with high reproducibility and under high pressure (up to 4000 psi).
4. Columns
- Columns are usually made of polished stainless steel, are between 50 and 300 mm long, and have an internal diameter between 2 and 5 mm.
- They are commonly filled with a stationary phase with a particle size of 3–10 µm.
- Columns with internal diameters of less than 2 mm are often called microbore columns.
- Ideally, the temperature of the mobile phase and the column should be kept constant during an analysis.
5. Detector
- The HPLC detector, located at the end of the column, detects the analytes as they elute from the chromatographic column.
- Commonly used detectors are UV-spectroscopy, fluorescence, mass-spectrometric and electrochemical detectors.
6. Data Collection Devices
- Signals from the detector may be collected on chart recorders or electronic integrators that vary in complexity and their ability to process, store and reprocess chromatographic data.
- The computer integrates the detector’s response to each component and places it into a chromatograph that is easy to read and interpret.
Applications of HPLC
The information that HPLC can obtain includes resolution, identification, and quantification of a compound. It also aids in chemical separation and purification. The other applications of HPLC include
Pharmaceutical Applications
- To control drug stability.
- Tablet dissolution study of pharmaceutical dosages form.
- Pharmaceutical quality control.
Environmental Applications
- Detection of phenolic compounds in drinking water.
- Bio-monitoring of pollutants.
Applications in Forensics
- Quantification of drugs in biological samples.
- Identification of steroids in blood, urine, etc.
- Forensic analysis of textile dyes.
- Determination of cocaine and other drugs of abuse in blood, urine, etc.
Food and Flavour
- Measurement of Quality of soft drinks and water.
- Sugar analysis in fruit juices.
- Analysis of polycyclic compounds in vegetables.
- Preservative analysis.
Applications in Clinical Tests
- Urine analysis, antibiotics analysis in blood.
- Analysis of bilirubin, biliverdin in hepatic disorders.
- Detection of endogenous Neuropeptides in the extracellular fluid of the brain etc.
Limitations
The limitation of using high-performance liquid chromatography (HPLC) is the following.
- HPLC is much more costly requires a large number of expensive organics.
- HPLC may have low sensitivity for certain compounds, and some cannot even be detected as they are irreversibly adsorbed.
- Complexity
- Volatile substances are much better to be separated by gas chromatography.
Frequently asked Questions
Q 1. What is liquid chromatography?
Liquid chromatography is one of the three main branches of chromatography. It involves a small volume of liquid sample placement into a tube packed with porous particles.
The individual components of the sample are transported along the column by a liquid moved with gravity.
The sample components are separated and then collected at the exit of this column.
Q 2. What is the principle of HPLC?
The principle of HPLC is based on analyte distribution between the mobile and stationary phases. It is crucial to remember that the sample’s different constituents elute at various times before the sample ingredients’ separation is achieved. The intermolecular interactions between sample and packaging materials molecules determine their time on-column.
Q 3. What are the types of HPLC?
There are four primary types of HPLC
1. Normal phase HPLC (effective method for separating phospholipid classes)
2. Reverse phase HPLC (the most common method used to separate compounds that have hydrophobic moieties)
3. Size-exclusion HPLC/molecular sieve chromatography (Used in large molecules/macromolecular complexes such as industrial polymers and proteins)
4. Ion-exchange HPLC (separates ions and polar molecules according to their ion exchanger.
Q 4. What are the four types of chromatography?
The four types of chromatography are
1. Liquid chromatography (test for pollution in water samples like lakes and rivers)
2. Gas chromatography (detect bombs and valuable in forensic investigations)
3. Thin-layer chromatography (used to check the purity of organic compounds such as the presence of insecticide or pesticide in foods)
4. Paper chromatography (uses a strip of paper in the stationary phase).
Q5. Why is high pressure needed in HPLC?
HPLC uses a moderate to high pressure to achieve the desired flow rate of the solvent through the chromatographic column as small particles have more excellent resistance to flow.
Q 6. What is the difference between isocratic and gradient?
Your application can be run in different ways – isocratic and gradient.
Isocratic is when the mobile phase mixture is consistent over the total testing time.
With a gradient, the compounding of the eluent mixture is changed during measurement, which significantly affects analyte retention. It can accelerate or decelerate the separation process.
Q 7. What solvent is used in HPLC?
Different solvents are used in HPLC, such as aqueous solvent (water) and organic solvent (methanol, acetonitrile, and propanol). To improve the chromatographic peak shape, acids such as acetic acid, formic acid, and trifluoroacetic acid can be used.
Q 8. What is the difference between UV and PDA detectors?
The PDA and UV are both absorbance detectors, which provide sensitivity for light-absorbing compounds. The UV detector is most commonly used for HPLC analysis.
The UV absorbance differs on the wavelength used, so it is essential to choose the right wavelength based on the type of analyte. On the other hand, the PDA detector adds a third dimension wavelength, which is a more convenient way of finding out the wavelength without repeating the analysis.
Q 9. What are the advantages of HPLC?
The advantages of HPLC are as follows:
1. It can test both raw materials and finished products.
2. It can reverse engineer formulations.
3. It helps solve product failure problems.
4. It can detect contaminants and other impurities.
5. It can perform competitor product analysis.
6. It can determine product stability and shelf life.
7. The testing can be done even with just a small sample size.
8. It enables you to modify the testing depending on the needed quantification level.
9. The results it produced are reliable.
10. It helps develop better products.
11. It lets you gain a better understanding of the competitor’s products.
Q 10. What is Rf value?
In chromatography, the RF value pertains to the distance a particular component traveled divided by the distance traveled by the solvent front. In other words, it is the characteristic of the component which is helpful in the identification of the components.
Q 11. What are the two main types of chromatography?
There are different types of chromatography, but the two primary types are liquid chromatography and gas chromatography.
Q 12. What is retention time?
It is when a specific analyte comes out of the end of the column.
References:
- Malviya, Rishabha & Bansal, Vvipin & Pal, Om & Sharma, Pramod. (2010). High-performance liquid chromatography: A short review. Journal of Global Pharma Technology. 2. 22-26.
- https://microbenotes.com/high-performance-liquid-chromatography-hplc/
- https://www.chemguide.co.uk/analysis/chromatography/hplc.html
- https://www2.chemistry.msu.edu/courses/cem434/Swain_2015_Lecture%20Notes/HPLC%20Lecture1a.pdf
- https://www.nature.com/subjects/liquid-chromatography
- https://www.knauer.net/en/Systems-Solutions/Analytical-HPLC-UHPLC/HPLC-Basicsprinciples-and-parameters
- https://www.azom.com/article.aspx?ArticleID=8468
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5702474/

suggest Me about research topic in cancer plzz
Dear Rakesh, It’d be better if you first choose your priority area and specific type of cancer on which you want to do a research. Then searching related articles with specified keywords on internet search engines (like Google Scholar) or databases (like PUBMED) will help you out a lot for choosing the topic. I wish you a very good luck.
Clear cut information. Tq
thank u dada.. it will help us
I’m glad that it helped you Sushmita. Keep visiting 🙂
I think here tcd , ecd, flam ionization left ???
Nice HPLC information
thank you for this information…
Thank you for information…. It helps me for my presentation
Thank you…………
thank youuuu sir … it helps me a lot in my exam
this s very very informative web site, Dhurba… excellent efforts… n pricision on ur knowledge.. congratulations!!! keep it up…
Good infrmation
Written in simple way… easily understandable language… good work… thanks DRubaa
super sir
Very helpful sir
Thank-you for the given good information
Thanks sir
Sir it is very useful for me and anybody can understand easily from this presentation.
thanks-
The information that is available in this presentation is better for me.
This is very useful to me… Thank you sir….
simple,short and straight to the point.very imformative and concise.well done
simple,short and straight to the point.very informative and concise.well done
thanks for good information
nice brief description
Thank u sir
thnx a lot mr.dhurba…. your posts helped me a lot…thnx for making things a bit easier for me….:-)
Explanation is easily understood and straight to the point. Very helpful! Thank you!
thanks boss
Very helpful… Nicely done.?
hplc analytical test about information is best given this manual and think is given perfect
Nice write up in clear words , articulate and helpful
sir,can you give me more details about detectors used in HPLC?
Want to know that, what pore size column we use for synthetic tripeptides in hplc
Very Helpful..For Last Minute Preparation…Thank You!
Simple and precise. Very helpful
Hplc or GC ka ki full knowledge Hindi me kese pata kru frnz anyone help me plz…
Thank you dear its really helpd me lot to Understand
Nice information
Very nice information…easily understandable
It’s useful for me and am now little clear about HPLC
It’s very helpful to me sir and thanks
Hello sir, I want to know about hplc.what is this. Ye test kaise hota hai. Actually ye test Dr. Ne mere bete ko prescribe kiya hai
Sir can you add some more details about detector
thanks bro
..volatile gas is not use,but inert gases like nitrogen,argon,hydrogen is use as mobile phase in GC…
thank you so much!! This helps me 😀
Interesting information. Thanks.
Hi Sir!!!
Could we please establish a collaboration? In fact i am a PHD Student working on veterinary drugs in Cameroon
thank you so much
Very nice.
Sir thank you very much..