The vegetative cells of some bacteria may put them under a great deal of stress, especially in a certain environment wherein they are deprived of nutrients such as carbon and nitrogen.
As a result, they produce inactive form-endospore. This is their coping mechanism for them to survive even in an unfavorable environment.
Endospore-forming bacteria are usually found in the soil as well as in the aquatic environment. Some can be found in medical settings such as in the case of patients with tetanus, botulism, and gas gangrene. Some of them can also be the reason for food poisoning. Anthrax is also caused by endospore-forming bacteria.
A staining method for endospore was published by Dorner in 1922. In 1933, the procedure was modified by Shaeffer and Fulton. The modified process is simpler and faster. Today, the Shaeffer and Fulton method is commonly used to differentiate bacterial endospores from other vegetative cells. It is also used to differentiate spore-forming bacteria from non-spore forming. (1, 2, 3, and 4)
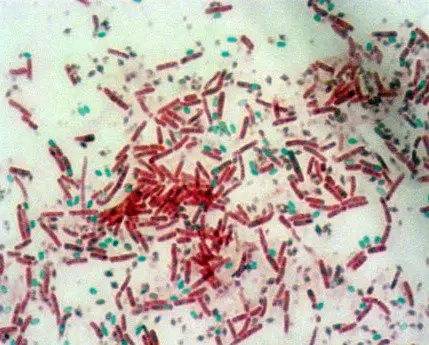
Image 1: Endospore staining; a microscopic view of the cells being studied for.
Picture Source: austincc.edu
What is the significance of endospore staining?
- It helps in classifying and differentiating bacteria.
- It is useful in the food and medicine industry, specifically in the can industry. It protects consumers by preventing food poisoning. (4, 5)
What are the principles of endospore staining?
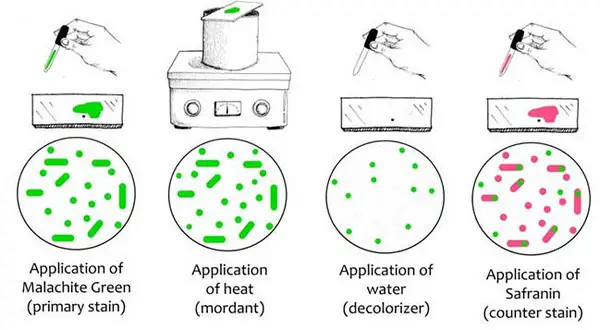
Image 2: Endospore staining procedure.
Picture Source: microbiologyinfo.com
A primary stain in the form of malachite green is used for staining endospores. It uses the heating process to act as a mordant as endospores have the ability to resist staining. Such a procedure does not require the use of decolorizer because malachite green binds to the cell and spore wall. The dye comes right out of the cell is washed thoroughly. However, once the spore wall is dyed, the color will lock in it.
Endospores are also resistant to de-staining. In other words, it has the ability to retain its primary dye even if the vegetative cells will lose the stain. To stain the decolorized vegetative cells, a counterstain should be added. Once you look at the cells under the microscope, you will notice the following:
- The color of the vegetative cell is pink or reddish.
- The vegetative cells containing endospores should be stained pink. On the other hand, the spores are viewed as green ellipses inside the cells.
- Mature endospores are seen as green ellipses too. They are not linked with vegetative bacteria. (4, 5, and 6)
How is Endospore Staining done?
The staining method for endospores is done in two ways: Dorner’s Method staining technique (traditional method) and Schaeffer-Fulton staining technique (modified method).
#1- Schaeffer-Dulton Technique (modified method)
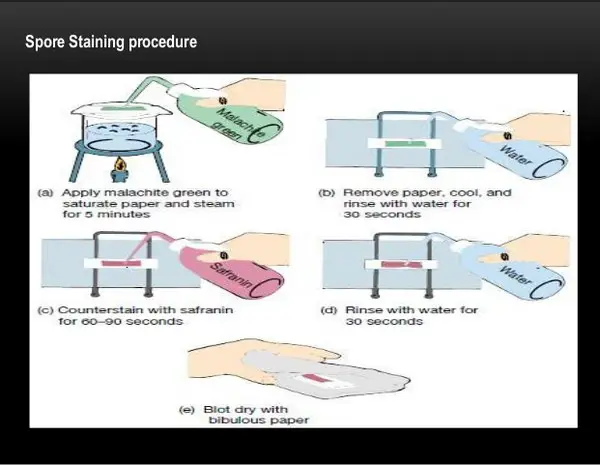
Image 3: An endospore staining procedure using the Schaeffer-Dulton Technique.
Picture Source: slidesharecdn.com
What do you need?
- Malachite green – it is used as the primary stain. The malachite green (0.5 grams) should be dissolved in water (100 ml).
- Distilled water – it acts as the decolorizing agent.
- Safranin – It acts as a counterstain. (5, 6, and 7)
Procedure
- Smear the sample to be studied at the center of the slide.
- Air dry the slide and heat fix.
- A blotting paper is put on the slide and the malachite green stain solution is pour on the slide.
- Place the slide on the heat until it evaporates. Boiling water or a Bunsen burner can be used to heat the slide.
- Heat, remove and re-heat the slide for about five minutes. Keep the blotting paper moist by adding drops of malachite green. The purpose of this step is to steam the slide and not to overheat it.
- Remove the slide and let it cool for a few minutes.
- Wash the slide. You can use distilled water or tap water whichever is available.
- Counterstaining is done using safranin.
- Rinse the slide and let it dry naturally.
- Examine the slide under the microscope. (6, 7, and 8)
Note: Endospores have a permeable barrier, which prevents the dye from staining the cell’s structure. For staining to be made possible, the barrier should be destroyed first.
The heat is used to destroy the barrier and enabling the dye to interact with petodoglycan. In other words, the heat acts as the mordant; a substance used together with the dye to completely fix in a particular material.
Once the heat fixing is done, the next step is to wash the slide using tap or distilled water, whichever is available. The purpose of rinsing the slide with water is to decolorize the slide. Malachite green can be washed off easily because it weakly binds to the endospore, but once it is completely locked in the wall of the spore, it cannot be washed off easily by water.
Endospores will retain the dye and it will be extremely hard to de-stain it. Once the initial washing is done, the next step is to apply safranin, which serves as the counterstain. It is done to counterstain the vegetative cells. As you notice, the colors used for primary and secondary staining are different.
It is purposely done that way so that the lab technician can easily differentiate the cells when viewing under the microscope. Under the microscope, the vegetative cells appear reddish to pinkish in color while the endospores are green in color when examined under the microscope. (9, 10)
#2 – Dorner’s Method
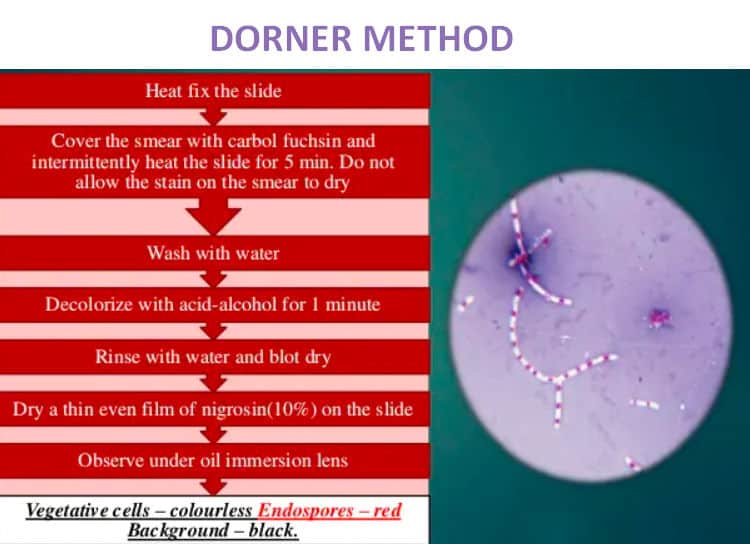
Image 4: An endospore staining method using the Dorner’s method.
Picture Source: generalmicroscience.com
What do you need?
- Carbolfuchsin stain
- Acid-alcohol, which acts as a decolorizing agent
- Nigrosin solution, which acts as a counterstain (2, 4, and 6)
Procedure
- Apply a smear on the slide.
- Let the slide dry naturally.
- Apply heat either by boiling or using a Bunsen burner.
- Cover the smear with a blotting paper and saturate using carbolfuschin for about five minutes. Make sure you avoid overheating the slide.
- Get rid of the blotting paper and let the slide dry out.
- Decolorize the slide using acid-alcohol and rinse with water.
- Counterstain by adding a drop of nigrosine.
- Let the slide dry out.
- Examine the slide under the microscope using oil immersion. (1, 6, and 9)
Note: When examined under the microscope, the endospores appear red in color.
Examples of endospore stain positive organisms
- Clostridium perfringens
- C. tetani
- C. botulinum
- Bacillus cereus
- Bacillus anthracis
- Sporosarcina spp
- Desulfotomaculum spp
- Sporolactobacillus spp
Examples of endospore stain negative organisms
- E. coli
- Salmonella spp (3, 6)
References
- http://www.austincc.edu/microbugz/endospore_stain.php
- https://en.wikipedia.org/wiki/Endospore_staining
- https://microbeonline.com/endospore-staining-principle-procedure-results/
- https://microbiologyinfo.com/endospore-staining-principle-reagents-procedure-and-result/
- https://www.microscopemaster.com/endospore-stain.html
- http://spot.pcc.edu/~jvolpe/b/bi234/lab/differentialTests/endospore_stain.htm
- http://www.asmscience.org/content/education/protocol/protocol.3112
- https://courses.lumenlearning.com/microbiology/chapter/staining-microscopic-specimens/
- https://milnepublishing.geneseo.edu/suny-microbiology-lab/chapter/differential-staining-techniques/
- https://www.scienceprofonline.com/microbiology/endospore-bacteria-stain-procedure.html
