Ions are one of the common words you will hear in the chemistry subject. They are formed from atoms and electrons by way of adding or removing one or more valence electrons, which could either be a positive or negative charge.
Negatively charged ions are referred to as anion while positively charged ions are cations.
In Newton’s law of attraction, opposite attracts. Therefore, cations and anions attract each other and form an ionic bond between them. (1, 2)
What are Cations?
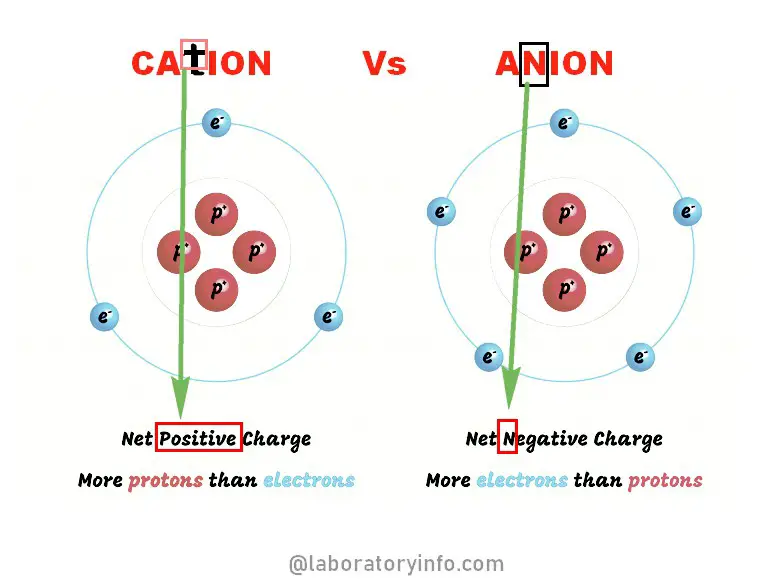
An atom in its neutral state has an equal number of protons and electrons. However, if it donates electrons (negatively charged), it is left with protons (positively charged), thus enabling the formation of cations to attain noble gas configuration.
During electrolysis, cations are attracted to the cathode (a negatively charged). The majority of metals are cations. A cation is derived from the Greek word “kata” meaning “down.”
Some of the common cations are hydrogen, calcium, and potassium. How much electron is lost as well as the charge of the ion is shown after the chemical symbol. (2, 3)
What are Anions?
Anions are negatively charged ions. They can be monovalent (can combine with single hydrogen ion) or bivalent (can form two chemical bonds).
Such a type of ion is attracted to anode (positively charged) during electrolysis. If non-metals are reduced or ionized, they usually form polar compounds. In other words, when a non-metal gains electrons, anions are formed. Examples of anions are chlorine, iodide, and hydroxide.
One distinct characteristic of the anion is that, it has more electrons than protons. The term anion is derived from the Greek word, “ano” which means “up”.
Anions are formed when one or more electron is gained from another low-affinity atom. (1, 3)
| Point of Comparison | Cations | Anions |
|---|---|---|
| Meaning | Cations are atom or molecule that has more protons than electrons, making it a positively charged ion. (3, 4) | Anions are atom/molecules that have more electrons than protons, hence, they are negatively charged ions. |
| Examples | Iron, Sodium, Ammonium, Silver, hydronium, and Lead | Chloride, bromide sulfate, Fluoride, nitride, hydride, and iodide. (8) |
| Charge | Positively charged | Negatively charged |
| Electrode attracted to | Cathode (negative) | Anode (positive) |
| Formed by | Metal atoms | Non-metal atoms |
| Electrolysis | They move in the direction of cathode (produces negatively charged ions) | They move in the direction of anode (produces positively charged ions) |
| Etymology | The Greek word “kata”, which means “down” | Greek word “ano” which means “up” |
| Electrons vs protons | Cations have more number of protons than electrons, which explains why it is positively charged ions. | Anions have more number of electrons than protons, which explains why it is negatively charged ions. (4) |
| How they are formed | Cations are formed when metals lose their electrons. | Anions are formed when non-metals gain electrons. |
| Charge indicator | A positively charged ion has a superscript + after the chemical formula. (5) | Anions are indicated with a superscript – after the chemical formula. |
| What happens during electrolysis | During electrolysis, positively charged ions (cations) move in the direction of cathode, hence, producing a negative charge. | During electrolysis, negatively charged ions (anions) in the direction of anode thereby producing positively charged ions. (5, 6) |
| Periodic table predication | If you look at the periodic table, you can easily predict if an element will form cations. Those always form cations are alkali metals, alkaline earth metals, and most other metals such as silver, iron, and nickel. (6, 7) | Looking at the position of an atom in the periodic table, you will be able to predict if an element forms anions. Those that typically form anions are halogens and most non-metals like carbon, sulfur, and oxygen. (7) |
| Rules when writing the formula of a compound | Cations are listed before the anions. Examples, when wring formula, are NaCl and MgCl. | The anions are listed after the cations. |
| Size | If you look at the diameter of cations, they are smaller than anions. (8) | Anions are bigger in diameter than cations. |
| Crystal lattice | They take up the space between two anions in the crystal lattice. | In the crystal lattice, anions occupy most of the space. |
| Organic ions | Cations that are organic are known as carbocations. | Anions that are organic are termed carbanions. |
| Compounds formed | To form an ionic bond, they combine with anions. | To form an ionic bond, they combine with cations. |
Examples of cations and anions:
| Cations | Anions |
|---|---|
| Aluminum | Hydride |
| Ammonium | Oxide |
| Barium | Fluoride |
| Calcium | Chloride |
| Chromium(II) | Sulfide |
| Chromium(III) | Bromide |
| Magnesium | Phosphate |
| Manganese(II) | Arsenate |
| Manganese(III) | Arsenite |
| Mercury(I) | Nitrate |
| Hydrogen | Sulfate |
| Hydronium | Hydrogen Sulfate |
| Lead(II) | Chlorate |
| Lithium | Bromate |
| Mercury(II) | Carbonate |
| Nitronium | Cyanide |
| Strontium | Formate |
| Tin(II) | |
| Tin(IV) | Oxalate |
| Zinc | Peroxide |
| Potassium | Hypochlorite |
| Silver | Dihydrogen phosphate |
| Sodium | Thiosulfate |
| Copper(I) | Perchlorate |
| Copper(II) | Bicarbonate |
| Iron(II) | Amide |
| Iron(III) | Nitrite |
The concept of cations and anions and its applications
Ions have the ability to conduct electricity through liquids or solutions. You might be wondering why there is a need to deeply and thoroughly study ions, specifically cations, and anions. Well, the main reason is these ions are responsible for the flow of electric current in the dry cell.
The current will flow towards the positive charge and moves to the opposing direction of the negative charge carrier. Basically, the electricity in the dry cell flows out of the cathode and flows into the anode. (8, 9, and 10)
What to keep in mind?
- Atoms are unstable under normal conditions.
- To make the atom stable, it should gain or lose one or more valence electrons for it to become an ion.
- The charge of the ion (positive or negative) will depend on the gain or loss.
- A net positive charge ion is called a cation.
- A net negative charge ion is called an anion.
- Electrostatic attraction is the term used for opposing charges, which is a result of ionic bonds between atoms or molecules.
- The stability of anion depends on the size of the atom and the location of electrons from the nucleus. The rule of the thumb is the more polarizable the atom, the more stable the anion. (3, 4, 7, and 10)
Conclusion
As you can see from the table above, cations and anions have so many differences. However, you have to keep in mind that the differences mainly depend on the ion’s electrical charge. Ions can be atoms or molecules that have lost or gained valence electrons either single or multiple.
Every ion is charged but they differ in the type of electrical charges attach to them. It is important to understand the differences between anions and cations, especially when using the periodic table.
Basically, it shows proof that opposite attracts and there is always a possibility that non-metals can get attracted to metals.
References
- https://biodifferences.com/difference-between-cation-and-anion.html
- https://microbenotes.com/cation-vs-anion/
- https://byjus.com/chemistry/anions-and-cations-difference/
- https://www.thoughtco.com/cation-and-an-anion-differences-606111
- https://www.technologynetworks.com/analysis/articles/cation-vs-anion-definition-chart-and-the-periodic-table-322863
- https://www.softschools.com/difference/anion_vs_cation/49/
- https://vivadifferences.com/10-differences-between-cations-and-anions-with-examples/
- https://sciencenotes.org/cations-and-anions/
- https://www.toppr.com/guides/biology/difference-between/cation-and-anion/
- https://www.diffen.com/difference/Anion_vs_Cation
