What are Lipids?
Lipids are organic compounds, nonpolar in nature – meaning soluble in nonpolar solvents. They are not soluble in water. In the human body, lipids are synthesized in the liver. Let us take a look at the structure of lipids.
Lipids are digested with the aid of a lipase enzyme, breaking down lipids into fatty acids and glycerol with the help of the bile in the liver. For lipids to be metabolized by the body it involves fatty acid oxidation to generate the energy needed to synthesize new lipids from minute molecules.
Lipid metabolism goes hand-in-hand with carbohydrate metabolism as glucose products and converted into lipids. Lipoproteins carry lipids to the bloodstream. (1, 2, and 3)

Image 1: The image above shows a biochemical structure of lipids.
Picture Source: wikimedia.org
Let us take a look at the composition of lipids:
Lipids have the most reduced form of hydrocarbons, which make them a perfect form of energy storage. When a hydrocarbon is metabolized it will oxidize and release a huge amount of energy.
In the fat cells, there’s an abundance of lipids in the form of triglycerides; an ester that formed from glycerol and fatty acids.

Image 2: The molecules of lipids as seen in the illustration above.
Picture Source: assignmentpoint.com

Image 3: A representation of the types and sources of lipids as seen on the image above.
Picture Source: assignmentpoint.com
Where do lipids come from?
When there’s an abundance of carbohydrates in your diet, the excess carbohydrates will be converted into triglycerides – synthesizing fatty acids from Acetyl-CoA through lipogenesis and happens in the cell’s endoplasmic reticulum.
The structure of lipids is outlined below:
- Lipids have three elements: carbon, oxygen, and hydrogen. However, they have a lower proportion of water when compared to other molecules like carbohydrates.
- Lipids don’t have repeating monomeric unit making them a polymer. Their composition is way different than proteins and polysaccharides.
- Lipids have two molecules – fatty acids and glycerol. Glycerol has three carbon atoms and a hydroxyl group attached to it. The remaining positions are occupied by hydrogen atoms. On the other hand, fatty acids have an acid group at one end of the molecule and a hydrocarbon chain.
- Lipids can be grouped as saturated or unsaturated. (2, 3, 4, and 5)

Image 4: Classification – Types and chemical properties of lipids.
What are the properties of lipids?
- Lipids are composed of oil and fat; high in energy and responsible for various bodily functions.
- In the body, they are stored in adipose tissues.
- They are heterogeneous compounds consist of hydrocarbon chains.
- They are energy-rich organic molecules providing life for various life processes.
- They are insoluble in water and soluble in non-polar solvents.
- They form the cell membrane; a barrier that divides cells from the external environment.
Lipids are fatty acid polymers consist of long, non-polar chain or hydrocarbon. They have a small polar region that contains oxygen. (4, 5, and 6)

Image 5: A chart comparing non-saponifiable and saponifiable lipids.
Picture Source: quizlet.com
Lipid classifications
There are two major classifications: non-saponifiable and saponifiable. The differences are discussed below:
- Non-saponifiable lipids – They are lipids that can’t be broken down into smaller molecules by hydrolysis. These include waxes, triglycerides, phospholipids, and sphingolipids.
- Saponifiable lipids – These are lipids that have one or more ester groups enabling the process of hydrolysis to take place but with the help of substances like acid, base, or enzymes.
Lipid categories
- Non-polar lipids – They are lipids used to store and fuel energy such as triglycerides.
- Polar lipids – They are used in membranes as they form a barrier with the external water environment. These include sphingolipids and glycerophospholipids. (6, 7)
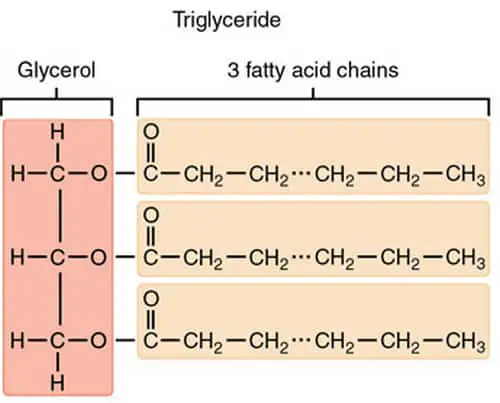
Image 6: A molecular presentation of simple lipids like fats, oil, and waxes.
Picture Source: alevelbiology.co.uk

Image 7: A structural presentation of complex lipids.
Picture Source: grossmont.edu

Image 8: Derived lipids include steroids, fatty acids, cholesterol, and some essential vitamins like A and D.
Picture Source: slideserve.com
Types of lipids
Simple lipids
These are esters of fatty acids containing various alcohols.
- Waxes – they have a high molecular weight monohydric alcohols. They are an ester of long-chain alcohol and a fatty acid with chains of the order of 12-34 carbon atoms in length.
- Fats and oils – fatty acids with glycerol, basically, fats and oil but in a liquid state. They are called triacylglycerols because they are primarily composed of three fatty acids combined with glycerol and trihydroxy alcohol. The difference between fats and oil is in their physical state at room temperature. A lipid is a fat if it is in solid-state. It is called an oil if it is in a liquid state.
Complex lipids
They contain ester of fatty acids in addition to fatty acid and alcohol.
- Glycolipids/glycosphingolipids – Characterized by components like sphingosine, fatty acid, and carbohydrate.
- Phospholipids – They contain fatty acids, alcohol, and residue of phosphoric acid. They have nitrogen-containing bases and other substituents.
- Other complex lipids – these include amino lipids, sulfolipids, and lipoproteins. (7, 8, and 9)
Derived lipids
They are the products of hydrolysis of both simple and compound lipids. These include glycerol, fatty acid, steroid derivatives, sphingosine, steroid hormones, hydrocarbons, lipid-soluble vitamins, ketone bodies, and mono and diacylglycerol, to name a few.
Neutral lipids
They are uncharged lipids. Examples are cholesterol, mono, di, and triacylglycerols, and cholesterol esters.
Miscellaneous lipids

These are compounds that share the same characteristics as lipids such as terpenes, pentacosone, hydrocarbons, squalene, and carotenoids. (1, 4, and 10)
Image 9: An image that represents the structure and functions of lipids.
Picture Source: biochemistryvd.files.wordpress.com
What are lipids used for ?
- Lipids are structural components of the cellular membrane.
- They provide energy in the body. They are stored in adipose tissues – the major source of energy. In humans, lipids are the best source of energy and provide most parts of calories. They contain a huge amount of energy in a little weight.
- They play an important role in the production of hormones. In fact, many hormones are lipids such as estrogen, cortisol, androgens, and prostaglandins, to name a few.
- They aid in the proper absorption of foods and healthy digestion.
- They take part in the signaling process.
- Some types of lipids form essential nutrients like vitamins D, A, E, and K. Vitamin A is good for the eyesight while vitamin D helps in the metabolism of calcium, a mineral essential for bone development and strength. Vitamin E prevents autoxidation of unsaturated lipids while vitamin K aids in the normal blood clotting process.
- Lipids can be used as bricks necessary for biological membrane construction thereby separating intracellular environment from extracellular one.
- On the plasma membrane, lipids act as receptors, antigens, and anchors for protein membranes. They have the ability to modify the structure and function of membrane enzymes.
- Some lipids regulate intracellular processes such as ceramides and diacylglycerol.
- Lipids reduce the possibility of body heat loss and aid in maintaining the ideal temperature of the body.
- Lipids are electrical insulation of axon of neurons covered by plasmatic membranes of Swann cells in the body’s nervous system, specifically in the peripheral area.
- Lipids facilitate some of the digestive tract processes. They depress gastric secretion and slows down gastric emptying. More so, they stimulate both pancreatic and biliary flow. (2, 4, 7, and 10)
Quick facts about lipids
- Lipid is derived from a Greek word meaning fat.
- Lipids are heterogeneous groups of compounds.
- Lipids are not polymers. They are tiny molecules.
- Lipids are the chief storage energy forms.
- They are useful in vital bodily processes, biochemical functions, and cellular structure.
- Lipids are among the major organic molecule groups.
- Too much or too little lipids are not good for human health.
- Too many saturated fat consumption increases the risk of cancer.
- Lipids aid in the absorption of essential vitamins like vitamins A, D, E, and K.
- Steroids are lipids too such as cortisol, chlorophyll, hormones, and cortisol. However, steroids used by athletes are not the same as natural lipid steroid, thus, causing damage to human health when used without supervision of health professional.
- Lipid imbalance in the body could lead to high cholesterol level, which increases the possibility of heart-related diseases.
- Lipids may have impact in some common diseases like asthma, rheumatoid arthritis, inflammatory diseases, Alzheimer’s disease, and some types of cancer.
- Lipid signaling plays an important role in cellular signaling/cell communication.
- The presence of lipid can be determined through an emulsion test; a wet chemistry wherein the sample is dissolved in alcohol and decanted into water. A white emulsion is achieved once the lipids are diluted which are not soluble in water.
- Too high level of lipids in the body causes hardening of the arteries, which can be extremely dangerous to human health. It increases the occurrence of heart-related conditions. (2, 4, 6, 9, and 10)
Note:
ICD-10 code for lipid panel – E78.5
Lipid guidelines 2020 – ESC/EAS Guidelines for the management of dyslipidaemias and ACC/AHA Guidelines
References
- https://www.news-medical.net/life-sciences/What-are-Lipids.aspx
- https://en.wikipedia.org/wiki/Lipid
- https://www.medicinenet.com/script/main/art.asp?articlekey=4168
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3995129/
- https://www.tuscany-diet.net/lipids/classification-functions/
- https://www.slideshare.net/mohdosman07/classification-of-lipids-42685487
- https://microbenotes.com/lipids-properties-structure-classification-and-functions/
- http://cyberlipid.gerli.com/description/classification-of-lipids/
- https://laney.edu/cheli-fossum/wp-content/uploads/sites/210/2019/04/Classification-of-Lipids.pdf
- https://proteopedia.org/wiki/index.php/Lipids:_structure_and_classification

