Ames test is a bacterial test used to identify carcinogens using mutagenicity in bacteria as the endpoint. This test is also called Salmonella typhimurium reverse mutation assay.
It is named after Bruce N Ames, a scientist who used to assess the potential carcinogenic effect of chemicals by using a particular strain of Salmonella typhimurium in the 1970s.
Ames test is a valid procedure of mutagenicity and is recognized by the government agencies and corporations.

Image 1: Ames test is used to find out the substance’s mutagenic and carcinogenic properties.
Picture Source: i.ytimg.com
Why is it important to determine mutagenicity?
Finding out the mutagenicity of a chemical substance is a significant part of testing the safety of a substance. Handling and exposure to a substance containing mutagenic chemicals can possess a health risk.
It could have a detrimental effect on the ova and sperm, which increases the possibility of mutation in offspring. Mutagenic chemicals can also increase the possibility of having cancer. (1, 2, 3, and 4)
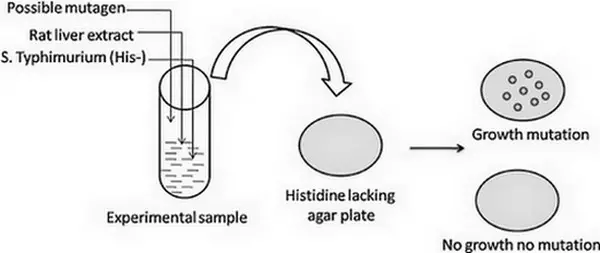
Image 2: The image shows how the Ames test is performed.
Picture Source: ars.els-cdn.com
What is the principle of the Ames test?
Ames test is derived on reverse mutation/back mutation principle. Hence, another term for the Ames test is bacterial reverse mutation assay or Salmonella typhimurium reverse mutation assay.
This test uses various strains of bacteria that may carry a mutation. One of the commonly used strains of bacteria is Salmonella Typhimurium.
It carries a mutation in a gene that contains histidine. It is an auxotrophic mutant. It loses its ability to mix histidine; a particular type of amino acid, by using the ingredients of the culture media. They are his strain and they need histidine in growth media.
Once you put His-salmonella in a medium that has certain chemicals, it will react with the medium thereby causing histidine encoding gene mutation. Hence, it regains the ability to blend histidine causing a reverse mutation.
Reverse mutation is made possible because of the chemical mutagen. Hence, it is safe to say that the Ames test determines the mutagenic property of a variety of chemicals. (3, 4, 5, 6, and 7)
Image 3: Colonies are formed in the plate indicating that the substance being tested for has mutagenic and carcinogenic properties.
Picture Source: acsh.org
How is the Ames test performed?
- Identify Salmonella typhimurium (auxotrophic strain) and isolate for histidine.
- A test suspension of Salmonella typhimurium should be prepared and placed in a plain buffer containing the chemical to be tested for.
- Add a few amounts of histidine. It is done to initiate bacterial growth. Only bacteria mutated has the ability to synthesize histidine after histidine is depleted. Such bacteria will form a colony.
- This time, get a control suspension of Salmonella Typhimurium minus the test chemicals.
- Incubate for approximately 20 minutes at a temperature of 37°C.
- Scatter the suspension on the agar plate.
- Incubate the plate for two days (48 hours) at 37°C.
- After the incubation period, inspect the plate for any colonies formed. The number of colonies formed is in proportion to the mutagenicity of chemicals. Hence, a chemical is said to be mutagens if there is a large number of colony formed on the test plate. (5, 6, 7, and 8)
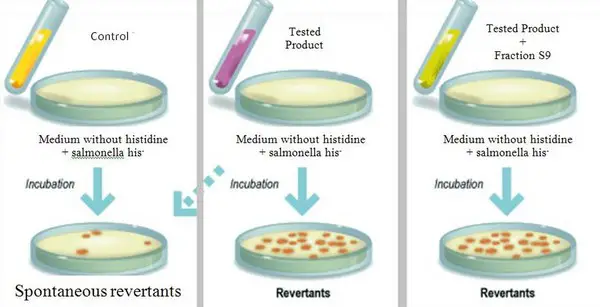
Image 4: The image shows the procedures used to perform Ames test.
Picture Source: researchgate.net
The applications of the Ames test
- It is done to screen mutagens that cause mutation, which have a carcinogenic effect on humans and animals. Examples of mutagenic and carcinogenic chemicals are those used as additives to foods such as AF-2, flavoring agent safrole, and anti-TB drug Isoniazid.
- It can detect mutants in a large population and highly sensitive bacteria.
- Ames test is primarily used to check for mutagenicity and not really the carcinogenic effect of a substance. However, it is found out that mutagens detected in the Ames test are also carcinogens.
- It can detect mutagenicity of environmental samples like dyes, drugs, cosmetics, reagents, pesticides, wastewater, and other substances. (8, 9, and 10)
Interpreting Results
The mutagenicity of a substance/chemical can be measured by observing the number of colonies formed. If there is a large number of colonies on the test plate, it means that the substance being tested for is a mutagen. If there are a few numbers of colonies on the plate, it may be caused by a spontaneous point mutation on the histidine encoding gene. (1, 4, and 6)
What are the advantages of the Ames test?
- Ames test is an easy, robust, and efficient bacterial assay.
- It is a fairly affordable test; an invaluable procedure for testing substances in the environment.
- It has the ability to detect suitable mutants even in a large bacterial population. (4, 6, and 7)
Are there any limitations?
- It is not the ideal model for human as it contains Salmonella typhimurium strain.
- Some carcinogenic substances do not test positive for the Ames test, especially substances in laboratory animals. (7, 9, and 10)
References
- https://en.wikipedia.org/wiki/Ames_test
- https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/ames-test
- http://www.onlinebiologynotes.com/ames-test-principle-procedure-and-application/
- https://www.eurofins.com.au/biopharma-services/testing-solutions/chemistry-toxicology/the-ames-test/
- http://www.biology-pages.info/A/AmesTest.html
- https://microbiologyinfo.com/ames-test/
- https://www.mun.ca/biology/scarr/4241_Ames_Test.html
- https://www.news-medical.net/life-sciences/The-Ames-Test.aspx
- https://www.creative-proteomics.com/services/ames-test.htm
- http://biotoxicity.com/index.php/ebpi-toxicity-tests/ames-tests
