Absorption and adsorption are two processes that are often used interchangeably. Although both have similarities, they differ in many ways. In this article, you will understand absorption and adsorption in their very sense.
What is Absorption?
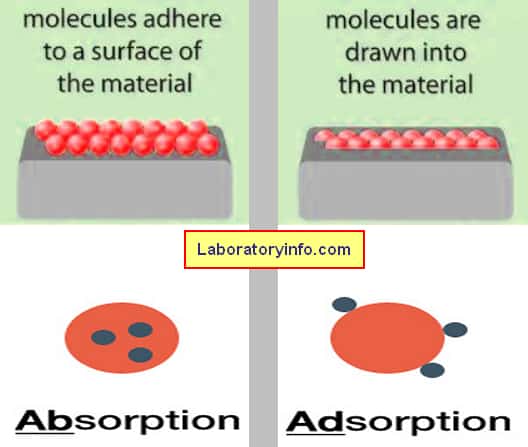
It pertains to a chemical or physical event where the substance’s atom, ion, or molecule binds into another substance.
The absorbate is the substance being absorbed while the substance that absorbs is referred to as absorbent. An example is a sponge and water.
The sponge has the ability to absorb water. In this scenario, the sponge is the absorbent and the absorbate is water. Other examples of absorption are:
- Skin absorption
- Intestinal absorption
- Absorption refrigerators (1, 2, 3, and 4)
What is Adsorption?
It is an occurrence in which the molecules of the substance stick on the surface of another substance (liquid/solid).
Adsorbate is the term used to describe the substance that gets adsorbed while adsorbent is the substance being adsorbed.
Interface refers to the surface where the entire process happens. Adsorption can either be in the form of physical or chemical. Examples of adsorption are:
- Adsorption of protein on biomaterials.
- Hydrogen gas occlusion on palladium.
- Packets of silica gel in new shoes.
- Activated charcoal in gas masks. (2, 3, 4, and 5)
The table below shows the brief yet detailed differences between absorption and adsorption.
| Point of reference | Absorption | Adsorption |
|---|---|---|
| Meaning/definition | Absorption is all about bringing in substances into the surface through the help of osmosis or diffusion. (3, 4) | Adsorption is all about adhesion. The molecules of the substance stick on the surface of other substances. |
| Components | There are two components: absorbate and absorbent. | There are two components: adsorbate and adsorbent. (5) |
| Principle | The absorption process is made possible because of the available space in the substance as well as the nature of the particle. (6, 7) | The process of adsorption is possible due to the vacant space of the adsorbent responsible for stimulating the adhesion of particles onto the available spaces. (7) |
| Types | There are two types: physical and chemical absorption.
|
|
| Exchange of heat | The exchange of heat relies on the endothermic process; the energy comes from the outside of the surface and it is only the absorption process that the energy of the absorbent increases. (5, 8) | It uses an exothermic process. The energy of the surface gradually declines to lead to a reduction in the surface’s residual forces. (9, 10) |
| Bonding | The absorbed materials stay in the absorbent and will not have any chemical reactions with the absorbent. (3, 5) | In the adsorption process, the adsorbed materials stay attached to the adsorbent with the aid of covalent bonds or Van der Wall’s forces. (6, 8) |
| Rate | The process takes place at a uniform rate. (8) | The adsorption process steadily increases until such time that it reaches balance |
| Temperature | The process does not rely on temperature. | Such a process is dependent on the temperature. |
| Concentration | After the process of absorption, the concentration of absorbate and absorbent remains the same. | The concentration is not the same in the adsorbate and adsorbent. Adsorbate is more concentrated on the surface when compared with the section of the adsorbent. |
| Separation | You can separate the absorbed materials based on their chemical interactions. | To separate the adsorbed materials, you need to pass a new substance through the adsorbent’s surface. However, this method will replace the previously adsorbed material. (6, 9, and 10) |
| Uses/applications |
|
|
| Phenomenon | Bulk phenomenon | Surface phenomenon |
| Examples |
|
|
Summary of the primary differences between absorption and adsorption.
- While adsorption and absorption sound similar are often used interchangeably, you have to remember that they are different processes.
- Absorption is when a substance is absorbed or taken by in bulk by another substance while adsorption is a surface phenomenon wherein a particular substance adheres to the surface of another substance.
- Absorption has a uniform reaction rate and endothermic process whereas adsorption has a steady reaction rate until such time that it reaches balance. The adsorption process is exothermic. When it comes to concentration, the concentration of the absorbed substance does not change. It remains the same throughout the medium.
- However, in adsorption, the adsorbed substance’s concentration changes. Temperature-wise, the absorption process is not affected. However, adsorption works well at a low temperature.
- When it comes to industries where absorption plays major roles, they include turbine inlet cooling, ice production, cold storage, and refrigerants. Adsorption is useful in water purification, air conditioning, chillers, and synthetic resin, to name a few.
So the next time you are going to use absorption and adsorption, you have to be extra careful not to interchange them. They are homophones but are totally different in meaning and application.
Both have important roles to play in various industries they are used to. The differences outlined in the table only go to show that even two very similar words can have huge differences. (3, 6, and 10)
