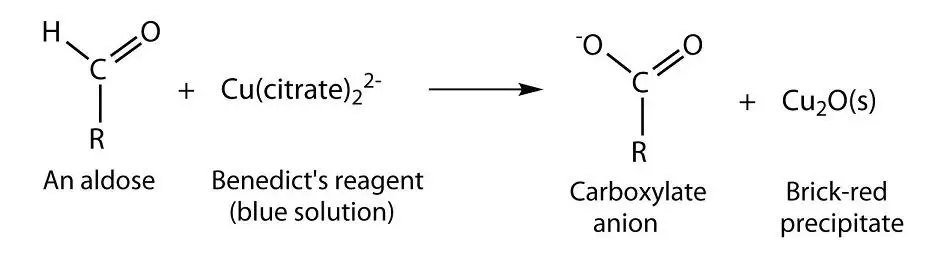
Benedict’s test is used as a simple test for reducing sugars. A reducing sugar is a carbohydrate possessing either a free aldehyde or free ketone functional group as part of its molecular structure. This includes all monosaccharides (eg. glucose, fructose, galactose) and many disaccharides, including lactose and maltose.
Benedict’s test is most commonly used to test for the presence of glucose in urine. Glucose found to be present in urine is an indication of Diabetes mellitus.
Principle of Benedict’s Test
Reducing sugars under alkaline condition tautomerise and form enediols. Enediols are powerful reducing agents. They can reduce cupric ions (Cu2+) to cuprous form (Cu+), which is responsible for the change in color of the reaction mixture.
This is the basis of Benedict’s test. When the conditions are carefully controlled, the colouration developed and the amount of precipitate formed (Cuprous oxide) depends upon the amount of reducing sugars present.
Composition and Preparation of Benedict’s reagent
One litre of Benedict’s Solution can be prepared from 100 g of anhydrous sodium carbonate, 173 g of sodium citrate and 17.3 g of copper(II) sulfate pentahydrate.
| Constitutent | Amount | Functions |
|---|---|---|
| Copper sulphate | 17.3 gm | Furnishes cupric ions (Cu++) |
| Sodium carbonate | 100 gm | Makes medium alkaline |
| Sodium citrate | 173 gm | Complexes with the copper (II) ions so that they do not deteriorate to copper(I) ions during storage |
| Distilled water | Upto 1000 ml | Solvent |
Quality Checking : Benedict’s solution is blue in color. In order to check purity of Benedict’s solution take 5 ml of Benedict’s solution in test tube and heat it. If is does not change color, it means it is pure.
Procedure of Benedict’s Test
- Pipette 5 ml of Benedict’s reagent in a test tube (20x150mm).
- Add 8 drops of urine to the Benedict’s reagent.
- Heat carefully on a flame of a gas burner or place in a boiling water for 5-10 minutes.
- Cool under tap water or by placing in a beaker containing tap water.
- Observe the color change and precipitate formation and analyse the test result.
Result Interpretation of Benedict’s Test
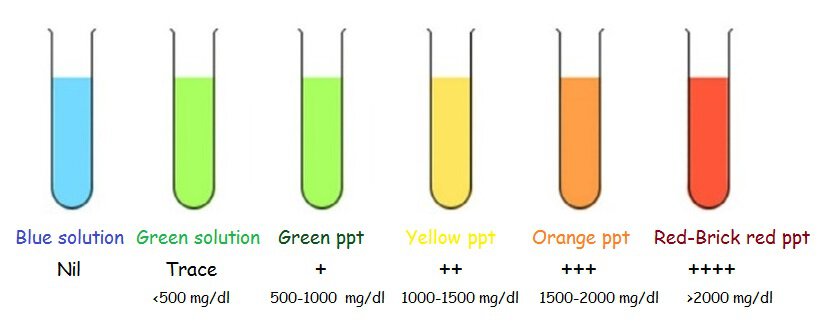
The colour of the mixture serves as a guide to the amount of sugar in the urine. Remove the tubes and examine the solution in each tube for precipitate and change of colour. Report the sugar concentration as follows:
| Color | Approximate glucose mg/dl | Indication |
|---|---|---|
| Blue solution | Nil | |
| Green solution | <500 mg/dl | Trace |
| Green ppt | 500-1000 mg/dl | + |
| Yellow ppt | 1000-1500 mg/dl | ++ |
| Orange ppt | 1500-2000 mg/dl | +++ |
| Red to Brick red ppt | >2000 mg/dl | ++++ |
Factors affecting Benedict’s Test
False positive reactions may also be obtained if certain drugs are present, e.g. salicylates, penicillin, streptomycin, isoniazid, and p-aminosalicyclic acid.
Chemicals present in a concentrated urine which may reduce Benedict’s reaction include creatinine, urate, and ascorbic acid (reduction is slight).

Amazing…..I m fan of this app…..wow.Well done Dhurba giri
Elaborated and is very useful for the beginners in medical lab technology
This cleared all my doubts….extremely useful!thank you
This was helpful to me.