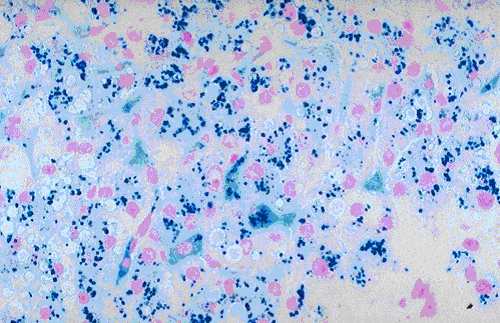
Hemosiderin is present in tissues as intracellular pigment. It contains iron in the form of ferric hydroxide that is bond to a protein framework.
Principle
The reaction occurs with the treatment of tissue sections with acid ferrocyanide solution. Any ferric ion (Fe3+) in the tissue combines with ferrocyanide and results in the formaion of a bright blue pigment called “prussian blue” or ferric ferrocyanide.
Fixation
Avoid the use of acid fixatives. Chromates will also interfare with the preservation of iron.
Staining solution
- 1% aqueous potassium ferrocyanide = 20 ml
- 2% aqueous hydrochloric acid = 20 ml
- Mix both
Procedure
- Deparaffinize and bring the sections to water.
- Treat the sections with freshly prepared acid ferrocyanide solution for 10-30 minutes.
- Wash well in distilled water.
- Lightly stain the nuclei with 0.5% aqueous neutral red or 0.1% nuclear fast red.
- Wash rapidly in distilled water.
- Dehydrate, clear and mount.
Result and Interpretation
- Ferric ion = blue
- Nuclei = red
- Background = pink