Syphilis is sexually transmitted (venereal) disease caused by spirochete Treponema pallidum. As the organism cannot be cultured in artificial media, the diagnosis of syphilis depends upon the correlation of clinical data either with the demonstration of microorganism in the lesion or serological testing.
Serological procedures for syphilis include the following.:
- Treponemal tests: detect the antibodies to Treponema pallidum. eg. Fluorescent Treponema pallidum antibody absorption (FTA-ABS) and microhemagglutination Treponema pallidum MHA-TP).
- Non-treponemal tests: detect the antibodies produced in response to lipoidal material released from the damaged host cell. These antibodies are traditionally referred to as ‘REAGINS’. eg; Venereal Disease research laboratory (VDRL) and the rapid plasma reagin (RPR) tests.
Venereal Disease Research Laboratory (VDRL)
The Venereal disease research laboratory (VDRL) test is a non-treponemal microflocculation test which is used for screening of syphilis.
It detects the IgM and IgG antibodies to lipoidal material released from the damaged host cells, as well as to lipoprotein-like material and possibly cardiolipin released from the treponemes.
Principle
The presence of lipoidal antibodies in patient’s serum or cerobrospinal fluid (CSF) is detected by using non-specific antigen, suspended in buffered saline solution. The cardiolipin antigen is an alcoholic solution composed of 0.03% cardiolipin, 0.21% lecithin and 0.9% cholesterol.
The heat inactivated (to destroy complement) serum or CSF is mixed with VDRL antigen. If the specimen contains reagin, flocculation occurs which can be observed using microscope. Non-reactive specimens appear as homogeneous suspension.
Specimens
Only serum and CSF are appropriate specimens for VDRL testing. Serum should be heated to 56 degree celcius for 30 minutes to destroy the complement, while no heat treatment is requied for CSF.
Qualitative method
Procedure
- Bring the VDRL antigen suspension, controls and samples to room temperature.
- Pipette one drop (50 µl) of the test specimen, positive and negative controls onto separate reaction circles of the disposable slide.
- Add one drop of well-mixed VDRL antigen next to the test specimen, positive control and negative control.
- Using a mixing stick mix the test specimen and the VDRL reagent thoroughly spreading uniformly over the entire reaction circle.
- Rotate the slide gently and continuously either manually or on a mechanical rotor at 180 r.p.m.
- Observe for flocculation microscopically at 8 minutes.
Interpretation
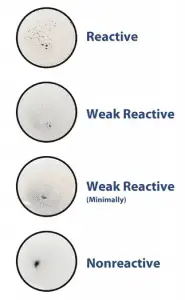
Reactive: Indicated by large or small aggregates in the center or the periphery of the test circle.
Non-reactive: Indicated by a smooth, even light gray appearance with no aggregates visible.
Semi-qualitative method
- Using isotonic saline prepare serial dilutions of the test sample positive in the qualitative method 1:2, 1:4, 1:8, 1:16,1:32, 1:64, 1:128 and so on as follows :
- For each specimen to be tested, add 100 µL of 0.9% saline into test tubes numbered 1 to 5.
- Add 100 µL of specimen onto test tube 1.
- Mix the mixture. Avoid formation of bubbles.
- Transfer 100 µL of mixed sample from tube 1 to 2.
- Repeat this serial dilution procedure in tube 3 to 4, and then 5. Dispose 100 µL from test tube 5 after mixing.
- Tubes 1 to 5 now represent a dilution series as follows:
Tube Number 1 2 3 4 5 Dilution 01:02 01:04 01:08 01:16 01:32
- Perform the qualitative test procedure using each dilution as test specimen.
Interpretation
The titre is reported as the reciprocal of the highest dilution, which shows a positive test result.
Limitations
- Biological false-positive reactions can occur with cardiolipin antigens, mainly in specimens from persons who abuse drugs; who have diseases such as lupus erythematosus, mononucleosis, malaria, leprosy or viral pneumonia; or who have recently been immunized.
- Reactive specimens should be subjected to further serologic study, including quantitation, whenever feasible.
- A prozone reaction may occur. In a prozone reaction, reactivity with undiluted serum is inhibited. The prozone phenomenon may be suspected when a specimen produces only a weakly reactive or a rough nonreactive result in a qualitative test.
- The VDRL may be reactive in persons from areas where yaws is endemic. As a rule, residual titers from these infections will be <1:8.
- There will not be a detectable immune response for 14-21 days after exposure. This test is most useful during the secondary stage of syphilis when the presence of reagin peaks, with typical results >1:32. It is less sensitive to primary syphilis, although there is a low level of <1:16 in about 80% of those who come for medical intervention in the primary stage.
Syphilis is sexually transmitted (venereal) disease caused by spirochete Treponema pallidum. As the organism cannot be cultured in artificial media, the diagnosis of syphilis depends upon the correlation of clinical data either with the demonstration of microorganism in the lesion or serological testing.
Serological procedures for syphilis include the following.:
- Treponemal tests: detect the antibodies to Treponema pallidum. eg. Fluorescent Treponema pallidum antibody absorption (FTA-ABS) and microhemagglutination Treponema pallidum MHA-TP).
- Non-treponemal tests: detect the antibodies produced in response to lipoidal material released from the damaged host cell. These antibodies are traditionally referred to as ‘REAGINS’. eg; Venereal Disease research laboratory (VDRL) and the rapid plasma reagin (RPR) tests.
Venereal Disease Research Laboratory (VDRL)
The Venereal disease research laboratory (VDRL) test is a non-treponemal microflocculation test which is used for screening of syphilis.
It detects the IgM and IgG antibodies to lipoidal material released from the damaged host cells, as well as to lipoprotein-like material and possibly cardiolipin released from the treponemes.
Principle
The presence of lipoidal antibodies in patient’s serum or cerobrospinal fluid (CSF) is detected by using non-specific antigen, suspended in buffered saline solution. The cardiolipin antigen is an alcoholic solution composed of 0.03% cardiolipin, 0.21% lecithin and 0.9% cholesterol.
The heat inactivated (to destroy complement) serum or CSF is mixed with VDRL antigen. If the specimen contains reagin, flocculation occurs which can be observed using microscope. Non-reactive specimens appear as homogeneous suspension.
Specimens
Only serum and CSF are appropriate specimens for VDRL testing. Serum should be heated to 56 degree celcius for 30 minutes to destroy the complement, while no heat treatment is requied for CSF.
Qualitative method
Procedure
- Bring the VDRL antigen suspension, controls and samples to room temperature.
- Pipette one drop (50 µl) of the test specimen, positive and negative controls onto separate reaction circles of the disposable slide.
- Add one drop of well-mixed VDRL antigen next to the test specimen, positive control and negative control.
- Using a mixing stick mix the test specimen and the VDRL reagent thoroughly spreading uniformly over the entire reaction circle.
- Rotate the slide gently and continuously either manually or on a mechanical rotor at 180 r.p.m.
- Observe for flocculation microscopically at 8 minutes.
Interpretation

Reactive: Indicated by large or small aggregates in the center or the periphery of the test circle.
Non-reactive: Indicated by a smooth, even light gray appearance with no aggregates visible.
Semi-qualitative method
- Using isotonic saline prepare serial dilutions of the test sample positive in the qualitative method 1:2, 1:4, 1:8, 1:16,1:32, 1:64, 1:128 and so on as follows :
- For each specimen to be tested, add 100 µL of 0.9% saline into test tubes numbered 1 to 5.
- Add 100 µL of specimen onto test tube 1.
- Mix the mixture. Avoid formation of bubbles.
- Transfer 100 µL of mixed sample from tube 1 to 2.
- Repeat this serial dilution procedure in tube 3 to 4, and then 5. Dispose 100 µL from test tube 5 after mixing.
- Tubes 1 to 5 now represent a dilution series as follows:
Tube Number 1 2 3 4 5 Dilution 01:02 01:04 01:08 01:16 01:32
- Perform the qualitative test procedure using each dilution as test specimen.
Interpretation
The titre is reported as the reciprocal of the highest dilution, which shows a positive test result.
Limitations
- Biological false-positive reactions can occur with cardiolipin antigens, mainly in specimens from persons who abuse drugs; who have diseases such as lupus erythematosus, mononucleosis, malaria, leprosy or viral pneumonia; or who have recently been immunized.
- Reactive specimens should be subjected to further serologic study, including quantitation, whenever feasible.
- A prozone reaction may occur. In a prozone reaction, reactivity with undiluted serum is inhibited. The prozone phenomenon may be suspected when a specimen produces only a weakly reactive or a rough nonreactive result in a qualitative test.
- The VDRL may be reactive in persons from areas where yaws is endemic. As a rule, residual titers from these infections will be <1:8.
- There will not be a detectable immune response for 14-21 days after exposure. This test is most useful during the secondary stage of syphilis when the presence of reagin peaks, with typical results >1:32. It is less sensitive to primary syphilis, although there is a low level of <1:16 in about 80% of those who come for medical intervention in the primary stage.

thank you sir
great note keep up good work
Keep it up
nice one need more of it
Nice one
What is the principle involved in the VDRL test?
a. Agglutination b. Precipitation C. Flocculation d. Opsonisation.